Dihydropteroate
Synthase
Lyra Hall '14 and Rina Petek '15
Contents:
I.
Introduction
Dihydropteroate
synthase (DHPS) is an enzyme involved in
the Bacillus anthracis
folate synthesis pathway. The enzyme has two binding pockets: one
which binds dihydropterin pyrophosphate (DHPP)
and one which binds p-amino benzoic acid(pABA).
DHPS catalyzes the
reaction which produces 7,8-dihydropteroate from
these two substrates. 
The
folate synthesis pathway is a crucial pathway for synthesizing
amino acids. Obviously, without amino acids, bacteria
cannot function, so this is an efficient method to inhibit bacterial
growth. In the past, the pABA pocket of DPHS has been targeted using
sulfonamides.
However, due to high clinical usage of
sulfonamides, bacterial populations have built up resistance to this
method. The most logical way to combat this bacterial resistance is to
simply
target the enzyme's other binding site, called the pterin pocket.
Pterins
are defined as a
class of heterocyclic compounds which mimic
dihydropterin pyrophosphate (DHPP): one of the enzyme's two natural
substrates. The key pharmacophore elements of DHPP are the two
nitrogenous aromatic rings with an amino group and a carbonyl group
para to each other.
As
a point of interest, the
first molcule discovered to inhibit DHPS by binding to the pterin
binding site
was 6-(methylamino)-5-nitroisocytosine known as MANIC.
II.
General Structure
Dihydropteroate
synthase is made up of two identical monomers of a classic TIM barrel
structure,
which
signifies 8 alpha helices alternating with 8 beta sheets.
With the assistance of
flexible linker strands, these helices and sheets curve in on each
other to make a shape called a toroid, or doughnut.The beta sheets
form the
inner wall of the doughnut while the alpha helices form the outer wall.
The core of the doughnut contains no peptide backbone, but is filled
with side chains of hydrophobic amino acids.
III.
Structure of Pterin Pocket
The
pterin binding site is located in the TIM barrel.The key residues that
recognize the pterin substrate
(in our model, PtPP) through hydrogen bonding are Asn120,
Asp184, Lys220, and a water molecule.
Arg254
and His 256
hydrogen bond with the beta phosphate on PtPP.
Interestingly, two flexible
loops are highly conserved throughout DHPS analogs of different
bacterial species. (loop
1 and loop2)
Loop
2 is not well
characterized in our model, as the loops only become fully ordered in a
dimer, which
has yet to be crystallized. Loop 1 is hypothesized to provide a
conserved aspartate to contribute to the catalytic mechanism of the
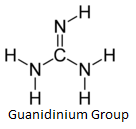
pABA binding site. Arg 68 on Loop 2 is thought to stabilize the pterin
site of the unbound enzyme due to the presence of a guanidinium group
which structurally resembles the pterin ring.
IV.
Structure of pABA Pocket
The
pABA binding site is poorly characterized since it is made up mostly of
difficult-to-characterize flexible loops. Nevertheless, Babaoglu et al
managed to crystallize a novel DHPS-product analog complex and were
able to infer some critical interactions that pABA makes in the barrel.
The
aromatic group of pABA has hydrophobic interactions with Lys220 and
Phe189, and the amine group
hydrogen bonds with Ser218.
V.
Advantages and Disadvantages of Targeting The Pterin Site for Drug
Design
There
are several advantages to targeting the pterin site rather than the
pABA site. First, all known mutations that confer resistance to sulfa
drugs occur within the pABA binding pocket. Therefore, inhibitory
compounds that bind to the pterin site can get around the most
prevalent method of bacterial resistance. Second, the pABA site is
composed of flexible loops, whereas the pterin site is severely
constrained by its structural integrity to the TIM barrel. In other
words, there is much less potential for bacterial mutation to the
pterin site that successfully maintains proper function of the protein.
Third, because of the tight restrictions placed by necessity upon it,
the pterin site is highly conserved across many species of bacteria, so
it presents an opportunity to create a drug that could potentially be
effective for a wide variety of bacterial-caused illnesses.
However,
there are some drawbacks. Pterin compounds tend to have poor
solubility. Due to their planar structure, they are much more stable in
a crystal lattice than they are in solution. Drugs must dissolve into
the body to be effective, so historically compounds with poor
solubility have been found to not make good drugs. Hevener et al say
this problem can potentially be addressed by adding anionic
functional groups. A second potential drawback to this pterin-based
method of design is that other opportunities may be missed out on. In
the field of drug design, a receptor-based approach to design is
preferred. A potential solution to this issue has already been
identified: one of the compounds Hevener et al discovered through
their
screening process is not a pterin compound, but successfully binds the
pterin pocket. Following this line of inquiry may lead to a much more
effective drug.
VI.
References
Hevener,
K.E., Yun, M., Qi,
J., Kerr, I.D., Babaoglu, K., Hurdle, J.G., Balakrishna, K., White, S.
W., and Lee, R.E. 2010. Structural Studies of Pterin-Based Inhibitors
of Dihyropteroate Synthase. J Med Chem. 53(1): 166-177.
Babaoglu,
K., Qi, J., Lee,
R.E., and White, S.W. Crystal Structure of 7,8-Dihydropteroate Synthase
from Bacillus anthracis: Mechanism and Novel Inhibitor Design.
Structure, Vol. 12, 1705–1717.
Adapted
from the Wikimedia Commons file
"File:Guanidine-2D.png"
http://en.wikipedia.org/wiki/File:Guanidine-2D.png#filelinks
Back to Top