S. cerevisiae Prp8
and Aar2
Kaylyn Stanton '17 and Emily Olson '17
Contents:
Model View:
I. Introduction
Before mRNA can be fully synthesized, splicing by the Spliceosome must
occur to remove introns from pre-mRNA. This molecule consists of five
small nuclear ribonucleoproteins (snRNPs) and multiple protein
complexes. Prp8 is one of these protein complexes, located in the U5
snRNP (Grainger et al 2005). The component is highly conserved across
most species, with over 60% conservation. It is also the largest
component in the spliceosome containing about 2400 amino acids and
varying between 230-250 kDa (Dlakic et al 2011). The Prp8 that we will
discuss is from Saccharomyces cerevisiae, commonly known as yeast.
Together with Brr2 and Snu114, Prp8 plays an important role in
spliceosome activation as it is located at the catalytic center of the
spliceosome.
Another component of the U5 snRNP is the protein Aar2. This protein is
required for the splicing of U3 precursors. Aar2 interacts with both the
RNaseH-like domain and the Jab1/MPN domain. These interactions are the
basis of the catalytic center of the spliceosome. Aar2 also acts to
regulate spliceosome activation by blocking the U4/U6 snRNA binding to
Prp8 and stalling spliceosome activation. Understanding the interactions
between Aar2 and Prp8 are essential to understand the structure and
function of the entire spliceosome.
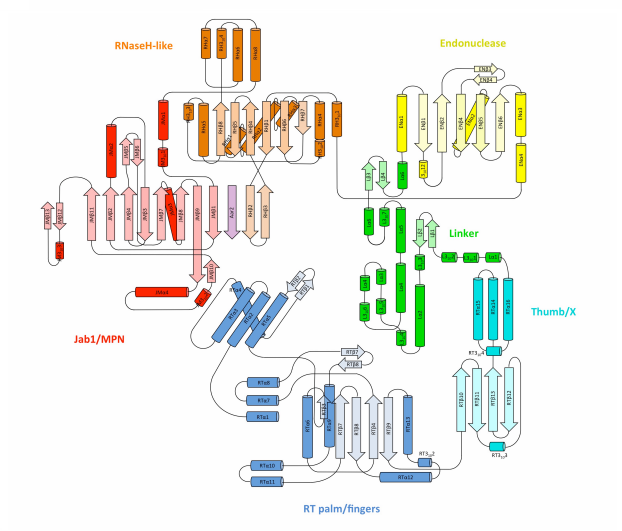
II. General Structure
Prp8 is comprised of
, however the first component, the N-terminal Domain, is not
shown. The six other domains are: 1. Reverse Transcriptase (RT)
Finger/Palm Domain, 2. RT Thumb/X Domain, 3. Linker, 4. Endonuclease
Domain, 5. RNaseH-like, and 6. Jab1/MPN Domain. Each of these domains
are detailed in the sections below.
is a smaller protein, containing 355 residues. It can be
broken up into the N-terminal Domain and the C-terminal Domain. The
C-terminal Domain is the region that interacts with Prp8.
Both the RNaseH-like domain and the Jab1/MPN domain interact with Aar2
by folding in to create a new, larger domain. This domain can be broken
down into the
and the
. These two domains interact through the
.
III. Large Polymerase-Like Domain
The large polymerase-like domain is made up of the
reverse transcriptase (RT) finger/palm region and the RT Thumb/X region.
In the
, the palm sub-domain forms a four-stranded antiparallel
beta-sheet, flanked by alpha-helices. The main motif in this domain is
, found in the loop between RTB7 and 8. Within this motif, there are
three aspartates that are incapable of binding divalent metal ions,
leading researchers to conclude that it has reverse transcriptase
activity. The RT Thumb/X region is made of four anti-parallel
beta-sheets (RTB10-RTB13) and a 3-alpha-helix bundle (RTa14-RTa16)
(Galej et al, 2013).
IV. Small Type II Restriction Endonuclease-Like Domain
The
of Prp8 comprises the small type II restriction endonuclease-like
domain. It is made up of five beta-strands: EnB1, EnB2, EnB4, EnB5, and
EnB6; and three alpha-helices: Ena1, Ena2, and Ena3. This domain forms a
cavity along with RNaseH-Like and Thumb domains that holds the catalytic
core of the group II intron RNA. While it is structurally similar to an
endonuclease domain of the influenza virus (which has catalytic
activity), mutations in the conserved elements of the endonuclease-like
fold does not affect the molecule. This suggests that this region is
conserved for structural and stability reasons (Galej et al, 2013).
IV. Prp8 and Aar2 Interaction
The
component directly interacts with the
Aar2 protein through its RNaseH-like domain and Jab1/MPN
domain. The C-terminal of the Aar2 protein forms a beta-sheet. This
sheet
together the beta-hairpin of RNase-like domain and the beta-barrel
of the Jab1/MPN domain.
The two proteins also interact through two alpha-helices of the
RNaseH-like region of Prp8 and the C-terminal helical domain of Aar2. An
arginine located at the tip of the RT-finger region interacts with an
asparagine and a leucine in the helical region of Aar2. Other contacts
between Prp8 and Aar2 occur through the linker.
occur between Aspartic Acid, Glycine, and Glutamic Acid of
Aar2 and Arginine, Glutamine, and another Arginine of the Prp8 linker,
respectively (Galej et al 2011).
VI. References
Dlakic, M., and A. Mushegian. "Prp8, the Pivotal
Protein of the Spliceosomal Catalytic Center, Evolved from a
Retroelement-encoded Reverse Transcriptase." RNA 17.5 (2011):
799-808. PMC. Web.
Galej, Wojciech P., Chris Oubridge, Andrew J.
Newman, and Kiyoshi Nagai. "Crystal Structure of Prp8 Reveals
Active Site Cavity of the Spliceosome." Nature 493.7434 (2013):
638-43. PMC. Web.
Grainger, Richard J., and Jean Beggs. "Prp8
Protein: At the Heart of the Spliceosome." RNA 11.5 (2005):
533-57. PMC. Web.
Back to Top