TeNT undergoes a pH-mediated conformational change
resulting from the proton pumping activity of ATPases in the
endosomal/vesicle membrane. This pH dependency is critical
to regulating TeNT's conformation to allow it to be
successfully transported to the interneuron and inhibit
vesicle release. Experimental data reveal that TeNT exhibits
a "compact conformation" when the pH is lower than 5.5.
However, TeNT enters an "extended conformation" when the pH
reaches higher than 6.5. The physiologically relevant change
in the maximum intramolecular distance of TeNT between these
pH states ranges from 125 A at pH 5.0 to approximately 140 A
at pH 8.0.
In context of its function in neurons, TeNT exists
in its extended conformation during retrograde axonal
transport, where the environment is a neutral pH. As it
encounters the acidic environment of synaptic vesicles near
the presynaptic interneuronal membrane, TeNT enters the
compact conformation, where the HC assists the LC in order
for TeNT to travel across the cell membrane [8].
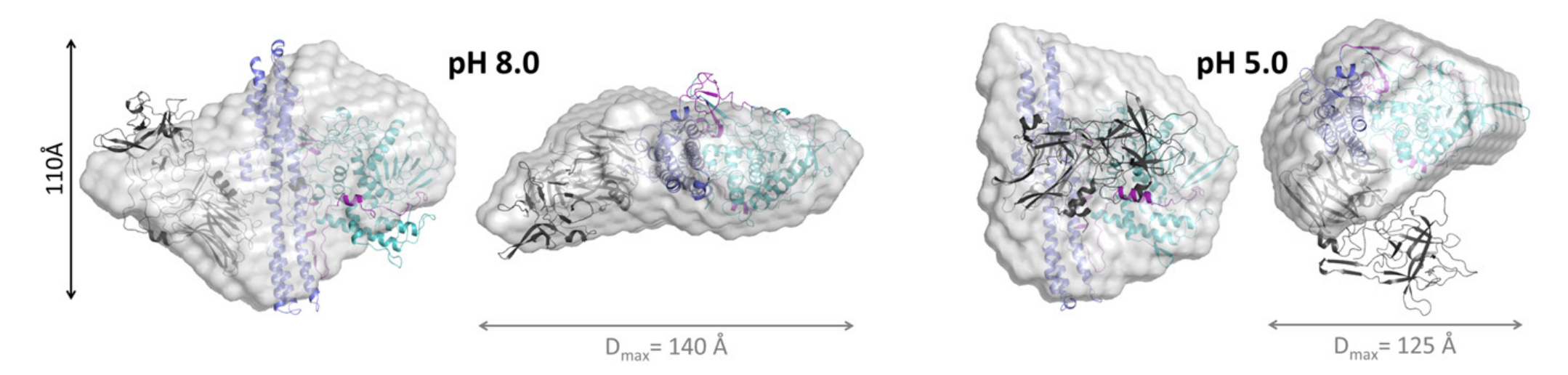
Figure 2.
Structure of extended (ph 8, left) and compact
(ph 5, right) conformational states of TeNT,
including change in intramolecular distance.[8]
Video 2. TeNT exhibits
pH-dependent dynamism. Extended and compact conformational
states define TeNT in basic and acidic pH microenvironments
respectively. [8].
V. References
[1] Clostridium tetani - microbewiki. (n.d.).
Retrieved December 16, 2018
[2]
Goonetilleke, A., and Harris, J. (2004). CLOSTRIDIAL
NEUROTOXINS. Journal of Neurology, Neurosurgery, and
Psychiatry, 75(Suppl 3), iii35�iii39.
[3]
Thwaites, C. L., and Loan, H. T. (2015). Eradication of
tetanus. British Medical Bulletin, 116(1), 69�77.
[4]
Synaptobrevin. (2018). In Wikipedia. Retrieved 02:19, December 18, 2018.
[5]
SNARE (protein). (2018). In Wikipedia. Retrieved 02:20, December 18, 2018.
[6]
Gamma-Aminobutyric acid. (2018). Wikipedia. Retrieved 02:20, December 18, 2018.
[7]
Glycine. (2018). In Wikipedia. Retrieved 02:21, December 18, 2018.
[8]
Masuyer G., Conrad J., and Stenmark P. (2017). The
structure of the tetanus toxin reveals pH-mediated domain
dynamics. EMBO reports. Vol. 18, No. 8, pp. 1306�1317,
2017.
[9]
Xi, A. P., Xu, Z. X., Liu, F. L., and Xu, Y. L. (2015).
Neuroprotective effects of
monosialotetrahexosylganglioside. Neural regeneration
research, 10(8), 1343-4.
Back to Top