Homo sapien
Serotonin Transporter (hSERT)
Phu Duong '21 and Allison Hector '21
Contents:
I. Introduction
View Type:
Depression
decreases the quality of life for more than 264 million people
worldwide [1]. The predominant pathophysiological hypothesis
attributes depression to decreased signalling of the monoamine
neurotransmitter serotonin (5-HT) at the neural synapse [2].
Serotonin modulates a broad range of brain and bodily functions from
sleep and behaviour to body temperature and reproduction. In
neurons, serotonin packages into vesicles. When an excitatory signal
reaches a presynaptic neuron, the serotonin-packed vesicles
translocate to the neuronal junction, releasing the neurotransmitter
into the synaptic cleft. Serotonin then binds to serotonin receptors
in the postsynaptic neuron activating an excitatory or inhibitory
cascade. As long as serotonin remains in the cleft, the signal
continues to propagate, until excess serotonin reuptake terminates
it [4].
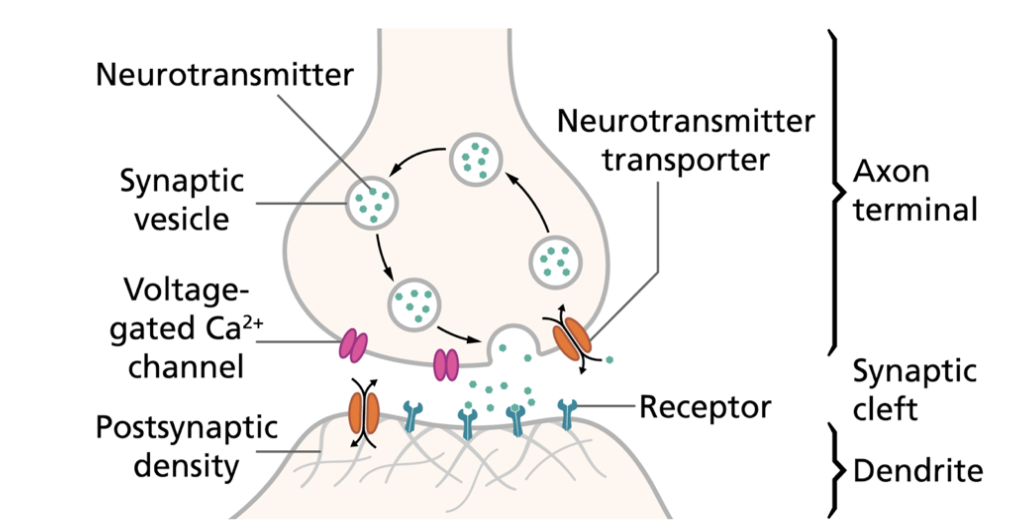
Fig
1. Illustration of the prototypical interneuron
signalling pathway. An
excitatory signal reaches the axon terminal, causing
release of neurotransmitters into the synaptic cleft.
Neurotransmitter ligands outward-facing receptors on
the surface of the postsynaptic neuron and thereby
induces a molecular or biochemical response.
Transporters at the presynaptic terminal remove excess
neurotransmitters from the cleft which terminates the
signal [4].
Fig 2.
Chemical structures of relevant hSERT
ligands. (A) The chemical
structure of the monoamine
neurotransmitter serotonin (5-HT) and its
protonated form, the substrate for hSERT
reuptake. (B) Chemical structure of the
selective-serotonin reuptake inhibitor
(SSRI) antidepressant (S)-citalopram.
Neurons require the Human Serotonin
Transporter (hSERT), a member of the
neurotransmitter sodium symporter family of
transport proteins, to expedite the
otherwise slow and energetically unfavorable
reuptake of 5-HT [5]. In its outward
rectifying conformation bound to sodium and
chloride, SERT initiates transport by first
binding a second sodium ion, followed by
protonated serotonin molecule (5-HT+).
Because of the high extracellular sodium
concentration maintained by the
Na+/K+-ATPase, the bound sodium readily
moves down its concentration gradient and
into the cell, driving serotonin transport
by inducing an inward rectifying
conformational change to the protein. Once
the protein flips inward to release the
second sodium ion and 5-HT+ into the
cytoplasm, intracellular potassium binds to
SERT, reverting it back to its outward
rectifying conformation, and then exits for
transport reinitiation (Fig
3) [6].
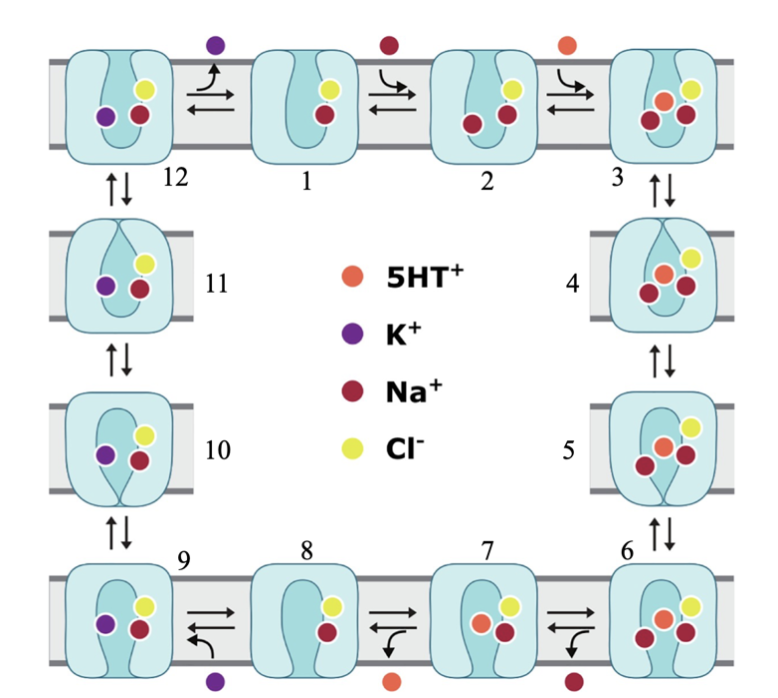
Fig
3. Schematic model of
hSERT transport.
(1-3) Na+ and Cl- bound
hSERT in its outward-open
conformation invites binding
of second Na+ and endogenous
5-HT+. (4-8) A series of
conformational changes
ensues until the inward-open
conformation is reached at
which time a Na+ and 5-HT+
is released into the
cytoplasm. (9-12)
Intracellular K+ ion binds
to the inward-facing
transporter, leading to
conformational reset of
hSERT as it exits the cell
[6].
II. General
Structure and Function
hSERT possesses twelve
transmembrane helices (
; helix colors correspond
to Fig
4) connected by
extra- and intracellular
loops. TM1-5 and TM6-10,
oppositely-oriented
structural repeats known
as a
fold, form the central
binding site for ligands
and ions. Within this
inverted-repeat, helices TM1,
TM2,
TM6,
and TM7
are thought to be the key
structural elements that
change conformation to
carry 5-HT across the
membrane, forming or
breaking molecular
interactions according to
gating mechanisms found at
the intra- and
extracellular vestibules
[5].
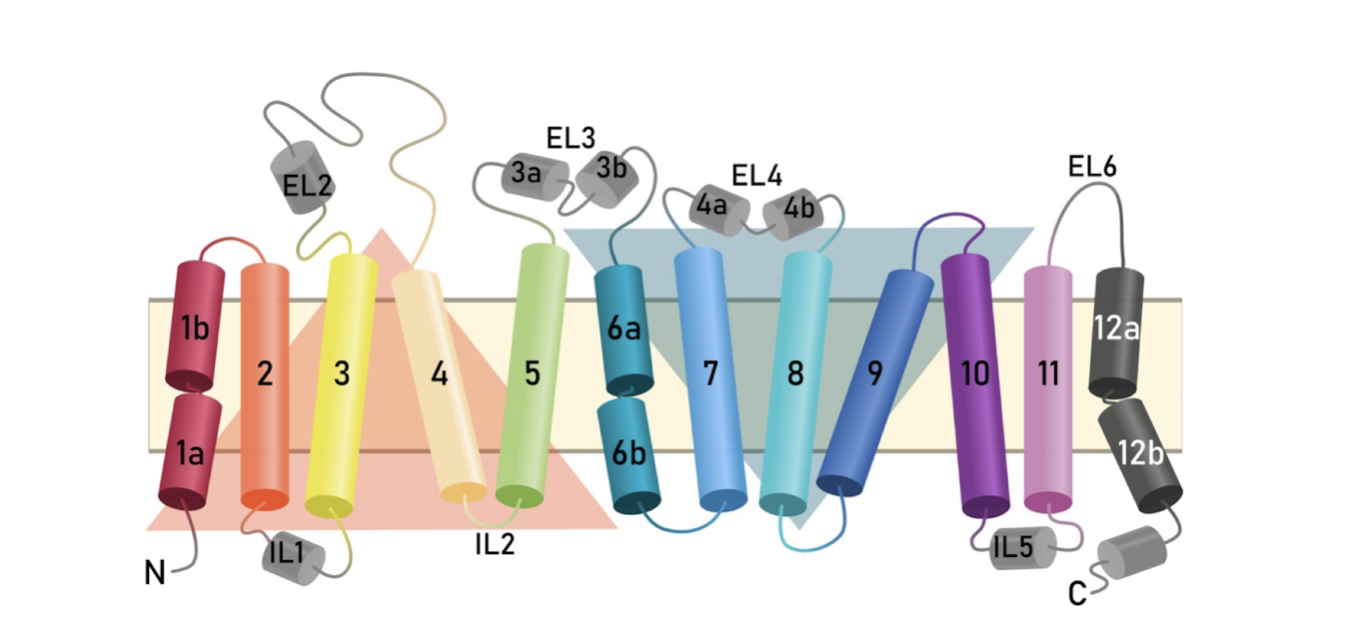
Fig
4.
Representation
of the
transmembrane
topology of
the human
SERT.
Intra- and
extracellular
loops connect
the twelve
membrane-spanning
helices. The
orange (TM1-5)
and blue
(TM6-10)
triangles
highlight the
inverted
structural
repeats known
as the LeuT
structural
fold [6].
III.
Endogenous
Substrate
Binding Sites
Ion
binding sites.
As
aforementioned
in the
Introduction,
SERT function
is sodium and
chloride-dependent.
The first
binding site,
formed by TM1,
TM6,
and TM7,
binds Na+ via
ion-dipole
interactions
with residue
backbone and
side chains.
The
ion binding
(not depicted)
is coordinated
by residues
from TM2, TM6,
and TM7. The
crucial second
ion, similar
to the first
Na+, forms
ion-dipole
interactions
with the
backbone and
side chains of
residues from
TM1
and TM8
[6][3].
Serotonin
binding at
orthosteric
site. As
of December
2019, there
are
no X-ray
structures of
hSERT bound to
serotonin.
Most recent
docking
experiments,
however, point
to several
potential
residues that
could interact
with
(Fig
2A)
based on what
is known about
the conserved
LeuT
structural
fold. Asp98
selectively
hydrogen bonds
with the
protonated
amine of
5-HT+. Ala173
contacts
aliphatic
regions with
hydrophobic
interactions,
while the
aromatic side
chains of Phe341
cradles the
indole ring.
Lastly, Ser439
engages in
H-bonding with
the hydroxyl
of the
neurotransmitter
[3].
IV.
Antidepressant
Binding Sites
Selective-Serotonin
Reuptake
Inhibitors
(SSRIs) are
drugs designed
to bind to
SERT with
higher
affinity than
serotonin,
competitively-inhibiting
serotonin
reuptake and
prolonging
serotonergic
signalling in
order to
decrease
depressive
symptoms in
patients [5].
The
antidepressant
(Fig 2B) occupies
both the
central and
allosteric
site of the
hSERT.
Antidepressant
binding at the
central site
sterically
inhibits
serotonin
binding, while
the
allosteric-bound
antidepressant
cooperates to
increase and
prolong
antidepressant-affinity
at the central
site. In the
binding site, the amine of (S)-citalopram, while interacting
with the
carboxylate of
Asp98, also
forms
cation-pi
interaction
with Tyr95
which may also
with its oxygen. The fluorophenyl is embedded within a
of hydrophobic and pi-pi interactions with surrounding aliphatic and
aromatic
residues,
while the
cyanophtalane
pi-stack with
nearby
phenylalanines.
At the
site, the cyanopthaline and fluorophenyl groups participate in
aromatic-specific
interactions
with Arg104
(cation-pi)
and Phe556
(aromatic-aromatic),
respectively
[3].
VI.
References
[1]
Depression.”
World Health
Organization,
World Health
Organization,
4 Dec. 2019,
https://www.who.int/news-room/fact-sheets/detail/depression
[2]
Cowen, Philip
J, and Michael
Browning.
“What Has
Serotonin to
Do with
Depression?”
World
Psychiatry :
Official
Journal of the
World
Psychiatric
Association
(WPA),
BlackWell
Publishing
Ltd, June
2015,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4471964/.
[3]Coleman,
Jonathan A.,
et al. “X-Ray
Structures and
Mechanism of
the Human
Serotonin
Transporter.”
Nature News,
Nature
Publishing
Group, 6 Apr.
2016,
https://www.nature.com/articles/nature17629.
[4]
“Action
Potentials and
Synapses.”
Queensland
Brain
Institute, 9
Nov. 2017,
https://qbi.uq.edu.au/brain-basics/brain/brain-physiology/action-potentials-and-synapses.
[5]
“IVB1.
Serotonin
Transporter: A
Representative
Reuptake
Pump.”
Serotonin
Transporter: A
Representative
Reuptake Pump,
https://web.williams.edu/imput/synapse/pages/IVB1.html.
[6]
Hellsberg,
Eva, et al. “A
Structural
Model of the
Human
Serotonin
Transporter in
an
Outward-Occluded
State.” A
Structural
Model of the
Human
Serotonin
Transporter in
an
Outward-Occluded
State, 2019,
doi:10.1101/637009.
Back
to Top