Homo Sapiens WT1
(Wilms' Tumor Protein) Zinc Fingers 2-4
Zachary Baker '24 and Freya Beinart '24
Contents:
I. Introduction
The protein
WT1, also known as Wilms Tumor Protein, is a transcription
factor studied primarily in humans for its role in development and as
the genomic origin of multiple mutation-based diseases and disorders.
This protein consists of ten exons, of which the first six code for the
N-terminal domain responsible for dimerization and the regulatory
effects of the protein while the last four code for zinc finger domains
1-4, respectively. The DNA binding activity of WT1 is mainly determined
by zinc fingers 2-4. While the mechanism by which WT1 regulates
transcription is not well understood, mutations within this gene have
been associated with a number of diseases and abnormalities such as
Denys-Drash Syndrome and Wilms Tumors. Under normal circumstances, WT1
plays a role in the development of various bodily systems in addition to
acting as a transcriptional activator of immune system cytokines and a
regulator of multiple cellular processes such as apoptosis. Within this
model, only
are shown.
II. General Structure and DNA Binding
The wt1 gene on chromosome 11p13 in Homo sapiens encodes a
zinc finger transcription factor. Alternative splicing between exons 9
and 10 of the wt1 gene may produce -KTS or +KTS isoforms in
which the amino acids lysine (K), threonine (T) and serine (S) may be
absent or present in the final transcript between zinc finger 3
and zinc finger 4 (Fig. 1). The protein shown is of the -KTS
isoform, where there is an absence of the three amino acids between
in zinc finger 3.
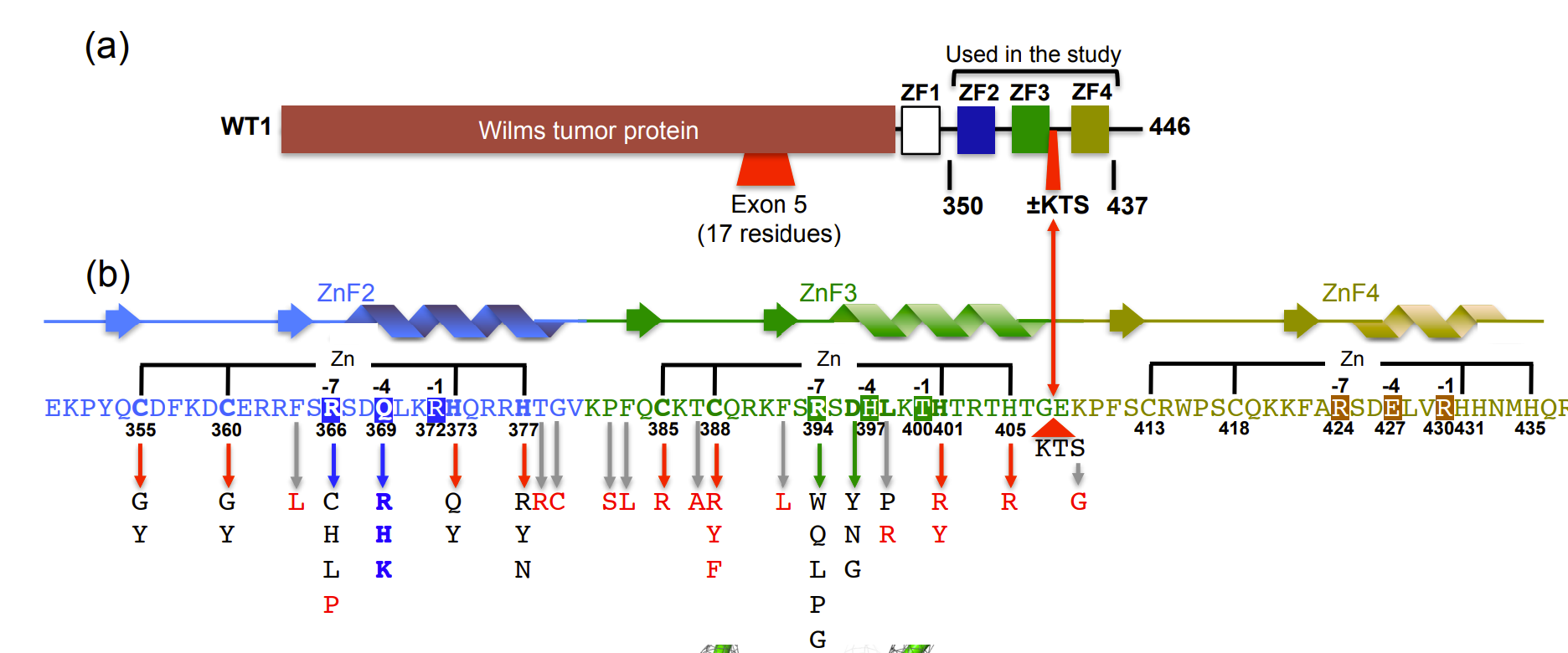
Figure 1. Schematic Representation
of WT1 (a) and Denys-Drash Syndrome associated amino acid
replacement (b). (Adapted from Hashimoto et al. 2016)
The zinc fingers (ZFs) each contain a
that stabilizes the tertiary structure of the protein by
binding both the alpha helix and antiparallel beta sheet. The ZFs
comprise a sequence of 30 amino acids which interact with a
recognition triplet of bases within the major groove of DNA. The WT1
consensus DNA binding sequence is 5 -GCG- (T/G)(G/A)G-G(C/A)G-3. In the
illustrated polymer, zinc finger 4 (ZF4) interacts with the 5' triplet
, zinc finger 3 (ZF3) interacts with the central triplet
,and zinc finger 2(ZF2) interacts with the 3' triplet
.
A 2016 study performed by Hashimoto et al. focused on the role of the
protein WT1 in the development of Denys-Drash Syndrome. Part of their
study observed -KTS isoform WT1 with the mutation
(Fig. 2), representing a change from glutamine to histidine
at position 369. This mutation is associated with the development of
DDS and is one of many that changes the genetic and epigenetic binding
specificity of ZF2, altering its effect on the host organism.
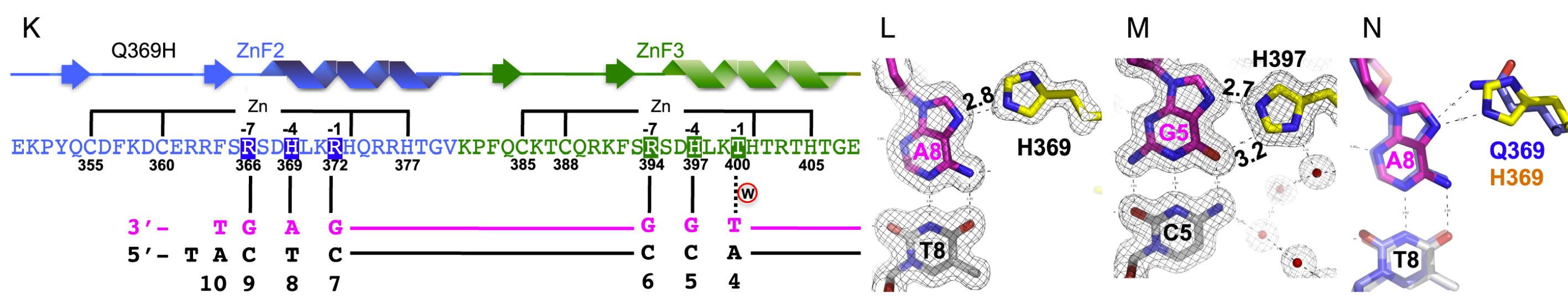
Figure 2. Schematic Representation
and DNA Binding of Q369H Mutant Protein. The Q369H mutation alters
the ZF2 binding domain of WT1, causing the protein to exhibit
slightly different DNA binding behaviors than the wild-type (K).
H639 interacts with Adenine 8 (L). H397 interacts with Guanine 5
(M). Superposition of Q369 (wild-type WT1) and H369 (mutant)
interactions with Adenine 8 (N). (Adapted from Hashimoto et al.
2016)
III. Canonical Effects
WT1 plays an important role in regulating the differentiation and
proliferation of nephroblasts and gonadal tissue in developing
fetuses. Additionally, WT1 has shown involvement in cellular
apoptosis, regulation of cytoskeletal architecture, and is responsible
for regulating expression of a wide variety of genes. The -KTS isoform
of the WT1 protein is often found at half the concentration of its
+KTS counterpart. The -KTS isoform, however, binds most stably to the
WT1 consensus sequence and seems primarily responsible for the
protein's role as a transcriptional regulator in development. There
are a number of exceptions however, such as regulation of the genes
for the vitamin D receptor and nephrin, in which the +KTS isoform is
the more efficient variant.
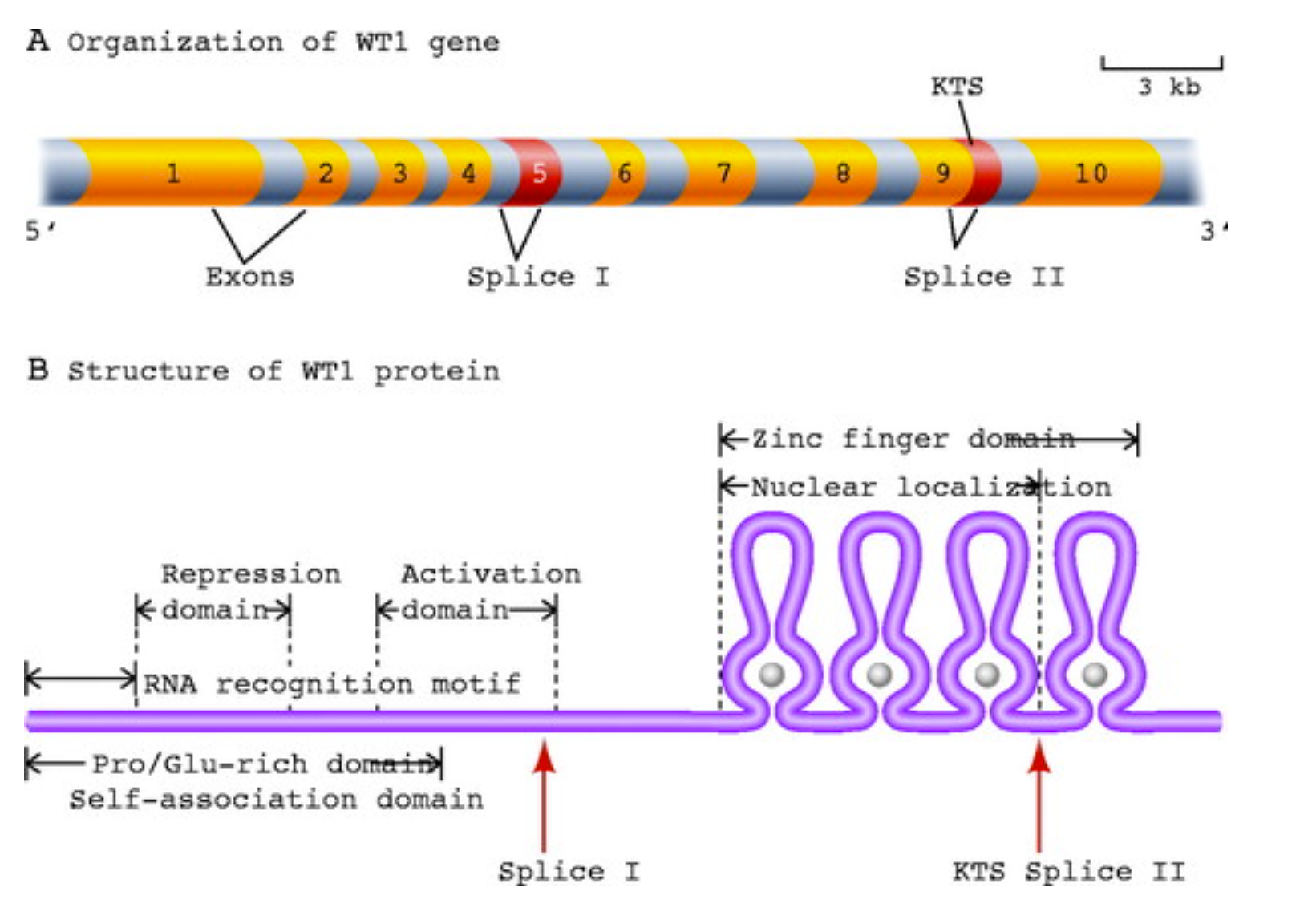
Figure 3. Genomic organization of wt1
exons and splice sites (A) and representation of WT1 protein domains
and splice sites (B). (Adapted from Scholz and Kirschner
2005)
WT1 has also been identified as a cis-acting transcriptional
activator of the immonosuppresive cytokine interleukin-10 (IL-10). The
WT1-responsive element within the promoter of the IL-10 gene allows
for binding of WT1 in addition to being necessary for stimulation of
the promoter by tumor necrosis factor alpha. In this case, the +KTS
isoform is much more efficient in stimulating activation of the IL-10
promoter.
Additionally, WT1 is suggested to be an important regulatory protein
in the development of neural tissue within the peripheral and
gastroenteric nervous systems. While the mechanism of this phenomenon
is not well understood, this relationship is insinuated by the gradual
decrease in cytoplasmic WT1 levels within sympathetic neuroblasts from
weeks 8 to 28 gestational age. It has also been found that both the
ganglion and chromaffin cells derived from these sympathetic
neuroblasts showed no WT1 expression in adults, strengthening the
likelihood of WT1's involvement in neural cell development.
IV. WT1 Related Abnormalities and Syndromes
Wilms Tumor is a malignant nephroblastoma, or kidney
cancer, that typically occurs in pediatric patients. Wilms Tumors are
often caused by a variety of mutations in the wt1 gene. Many
of these genetic mutations are associated with diseases such as
Denys-Drash Syndrome, Frasier Syndrome, and WAGR syndrome which
increase the predisposition for developing Wilms Tumors.
Denys-Drash Syndrome (DDS) is a disorder
characterized by abnormal development of the kidneys and genitalia in
pediatric patients. DDS is caused by multiple mutations in exon 8 or
exon 9 of the wt1 gene that encode ZF2 and ZF3, respectively.
The modelled protein is a mutant WT1 protein associated with DDS, as
the normal glutamine at position 369 is replaced by histidine (Q369H).
The mutations of the ZFs alter the sequence specificity, changing its
to discriminate some bases over others. The Q369H mutant has
a much higher affinity for purines than pyrimidines, with a higher
affinity for G over A.
Frasier syndrome (FS) is a kidney disease that
begins in early childhood with phenotypical characteristics such as
focal segmental glomerulosclerosis, gonadal dysgenesis, and high risk
of Wilms Tumors. FS is characterized by an altered ratio of -/+ KTS
alternative splicing isoforms resulting from specific intronic point
mutations.
WAGR syndrome (Wilms tumor, aniridia, genitourinary
malformations, and mental retardation) is characterized by newborn
aniridia, genital anomalies, obesity, and many other severe
abnormalities. WAGR syndrome can be caused by a deletion of several
genes on chromosome 11p13, including wt1.
V. References
Hashimoto, Hideharu, Xing Zhang, Yu Zheng,
Geoffrey G. Wilson, and Xiaodong Cheng. "Denys-Drash syndrome
associated WT1 glutamine 369 mutants have altered
sequence-preferences and altered responses to epigenetic
modifications." Nucleic acids research 44, no. 21 (2016):
10165-10176.
Lee, Sean Bong, and Daniel A. Haber. "Wilms
tumor and the WT1 gene." Experimental cell research 264,
no. 1 (2001): 74-99.
Scholz, Holger, and Karin M. Kirschner. "A
role for the Wilms' tumor protein WT1 in organ development." Physiology
20, no. 1 (2005): 54-59.
Sciesielski, Lina K., Karin M. Kirschner,
Holger Scholz, and Anja Bondke Persson. "Wilms' tumor protein Wt1
regulates the Interleukin-10 (IL-10) gene." FEBS letters 584,
no. 22 (2010): 4665-4671.
Parenti, Rosalba, Lidia Puzzo, Giada Maria
Vecchio, Lucia Gravina, Lucia Salvatorelli, Giuseppe Musumeci,
Enrico Vasquez, and Gaetano Magro. "Immunolocalization of Wilms'
Tumor protein (WT1) in developing human peripheral sympathetic and
gastroenteric nervous system." Acta histochemica 116, no.
1 (2014): 48-54.
Haber, Daniel A.,
Robert L. Sohn, Alan J. Buckler, Jerry Pelletier, Katherine M.
Call, and David E. Housman. "Alternative splicing and genomic
structure of the Wilms tumor gene WT1." Proceedings of the
National Academy of Sciences 88, no. 21 (1991):
9618-9622.
Klamt, Barbara, Ania
Koziell, Francis Poulat, Peter Wieacker, Peter Scambler,
Philippe Berta, and Manfred Gessler. "Frasier syndrome is
caused by defective alternative splicing of WT1 leading to an
altered ratio of WT1+/? KTS splice isoforms." Human
molecular genetics 7, no. 4 (1998): 709-714.
"WAGR Syndrome."
MedlinePlus. U.S. National Library of Medicine, September 8,
2020.
https://medlineplus.gov/genetics/condition/wagr-syndrome/.
Back to Top