Homo Sapiens
Surfactant Protein D
Eva Illuzzi '24 and Meera Chambers '24
Contents:
I. Introduction
Surfactant proteins (SP-A, B, C, D) are crucial to proper lung
function and development. The proteins play important roles in innate
immune response and reduction of alveolar surface tension in the
lungs. The surfactant proteins are generally grouped as SP-A and SP-D,
which aid in immune response, and SP-B and SP-C, which help lower
surface tension to prevent alveolar collapse. Surfactant protein D
(SP-D) is a member of the innate immune protein family, collectins,
and is hydrophilic in nature. SP-D has strong antiviral and
antimicrobial activity and enhances bacterial and viral clearance.
SP-D works to bind and opsonize possible pathogens which leads to the
facilitation of their elimination by other immune cells. SP-D can also
promote or inhibit immune cell activity by binding to other immune
defense cells.
II. General Structure
The SP-D protein visualized here:
is shown in its Y-shaped
trimer conformation and is made up of three nearly identical chains: chain
A , chain B,
and chain C . However,
SP-D is most often found as a dodecomer made up of four homotrimeric
subunits. The quaternary structure of SP-D consists of four trimers
bound at their respective N-termini to form an X-shaped structure
(Fig. 1).
The SP-D trimer is made up of monomers organized into four groups:
the N-terminal domain, the collagenous area , the neck region , and the C-terminal domain . The C-terminal carbohydrate recognition domain
(CRD) is responsible for the recognition of lung pathogens based on
surface lipopolysaccharides. The interaction between the CRD and
pathogen lipopolysaccharides targets the pathogen for clearance and
phagocytosis.
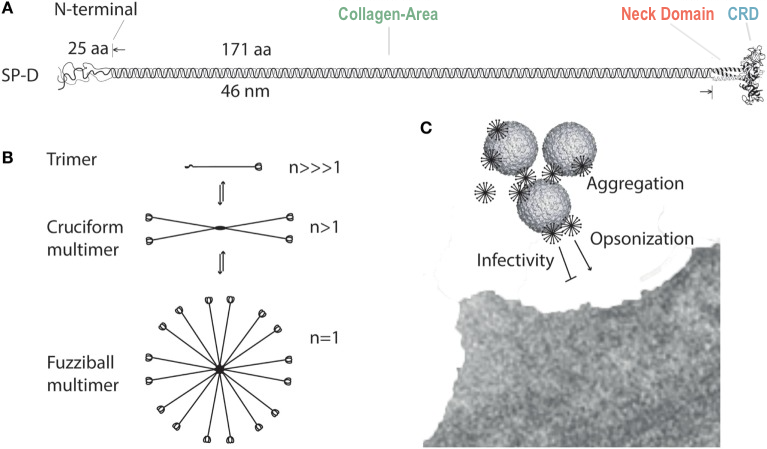
Figure 1. Surfactant protein D structure
and multimerization. Our protein model does not include the N-terminal
domain or full collagen-like domain. (Sorensen, 2018)
III. Protein Domains
N-terminal Domain [Shown in Figure 1.]
Each SP-D molecule is made up of trimeric subunits that interact at
the N-termini. Two conserved cysteine residues (positions 15 and 20)
participate in disulfide bridges that stabilize the trimer
interaction. The N terminal domain in SP-D is 25 residues in length
and located at one end of the protein.
Collagen Area [Binds at End of Neck Domain]
The largest domain of SP-D is a long, highly conserved, collagenous
domain in between the N-terminus and the neck of the protein. The
collagen domain is important for the spatial organization of the
protein and determines the separation of trimers. The highly conserved
nature of the collagen domain suggests that its role in spatial
organization is critical to SP-D function. Multiple post translational
modifications of SP-D target the collagen-like region and N-terminal
domain. These modifications include hydroxylations, glycosylations,
and nitrosylations and are important in the (in)ability of the SP-D
monomer to multimerize.
Neck Domain
The neck region of SP-D is an alpha helical coiled-coil, the function
of which is involved in SP-D trimerization. Various SP-D protein
recombinant studies have demonstrated the crucial role of the neck
region in trimerization of the protein. A protein recombinant
containing only the neck and CRD is still able to trimerize, while a
knockoff of the neck region inhibits protein trimerization. This
suggests that the neck region is critical in the formation of the SP-D
trimer.
Carbohydrate Recognition Domain (CRD)
The C-terminal end of the protein, also known as the carbohydrate
recognition domain (CRD), has a globular structure responsible for
ligand-binding. The three C-terminals are held together by the neck
region of the protein. However, the C-terminal end of the protein is
not fully threefold symmetrical which demonstrates flexibility of CRDs
in relation to the neck domain. There are two conserved consensus
sequences located at the two ends of the carbohydrate binding groove:
(Asp/Asn-Gly-Gly-Ser/Ala)
and (Arg/Lys-Ala/Val-Cys-Gly-Glu-X-Arg)
. These consensus sequences are critical to the recognition of specific
pathogens. The binding of the SP-D CRD to lipopolysaccharides is also
calcium dependent. Ca2+ ions
are bound to a small depression on the CRD, which is where
lipopolysaccharides bind.
IV. SP-D Real World Applications
Surfactant protein D can be used as a helpful biomarker for the
characterization of various respiratory diseases. In patients with
allergic asthma, increased severity of asthma can be indicated by an
increased concentration of SP-D serum levels (Benfante et al. 2016).
In addition, serum levels of SP-D can be used to differentiate between
respiratory diseases. In patients with severe pandemic influenza A
(H1N1) or severe COVID-19, plasma levels of SP-D were only elevated in
influenza patients (Choreno-Parra et al. 2021). This suggests that in
critically ill patients, serum levels of SP-D could be used to
elucidate the cause of respiratory ailments.
V. References
Benfante A, Battaglia S, Principe S, Di Mitri C, Paterno A, Spatafora
M, Scichilone N. 2016. Asthmatics with high levels of serum surfactant
protein D have more severe disease. Eur Respir J.
47(6):1864-1867. doi:10.1183/13993003.02142-2015.
Choreno-Parra JA, Jimenez-Alvarez LA, Ramirez-Martinez G,
Cruz-Lagunas A, Thapa M, Fernandez-Lopez LA, Carnalla-Cortes M,
Choreno-Parra EM, Mena-Hernandez L, Sandoval-Vega M, et al. 2021.
Expression of Surfactant Protein D Distinguishes Severe Pandemic
Influenza A(H1N1) from Coronavirus Disease 2019. J Infect Dis.
224(1):21-30. doi:10.1093/infdis/jiab113.
Clark HW, Mackay R-M, Deadman ME, Hood DW, Madsen J, Moxon ER,
Townsend JP, Reid KBM, Ahmed A, Shaw AJ, et al. 2016. Crystal
Structure of a Complex of Surfactant Protein D (SP-D) and Haemophilus
influenzae L ipopolysaccharide Reveals Shielding of Core Structures in
SP-D-Resistant Strains. Infect Immun. 84(5):1585-1592.
doi:10.1128/IAI.01239-15.
Crouch E, Persson A, Chang D, Heuser J. 1994. Molecular structure of
pulmonary surfactant protein D (SP-D). J Biol Chem.
269(25):17311-17319.
Crouch EC. 2000. Surfactant protein-D and pulmonary host defense. Respir
Res. 1(2):93-108. doi:10.1186/rr19.
Hakansson K, Lim NK, Hoppe HJ, Reid KB. 1999. Crystal structure of
the trimeric alpha-helical coiled-coil and the three lectin domains of
human lung surfactant protein D. Structure. 7(3):255-264.
doi:10.1016/s0969-2126(99)80036-7.
Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF,
Bernal AL, Reid KBM, Madan T, Chakraborty T. 2006. Surfactant proteins
SP-A and SP-D: structure, function and receptors. Mol Immunol.
43(9):1293-1315. doi:10.1016/j.molimm.2005.08.004.
Kovacs H, O'Ddonoghue SI, Hoppe H-J, Comfort D, Reid KBM, Campbell
lain D, Nilges M. 2002. Solution structure of the coiled-coil
trimerization domain from lung surfactant protein D. J Biomol NMR.
24(2):89-102. doi:10.1023/a:1020980006628.
Sorensen GL. 2018. Surfactant Protein D in Respiratory and
Non-Respiratory Diseases. Front Med. 5:18.
doi:10.3389/fmed.2018.00018.
Wang L, Brauner JW, Mao G, Crouch E, Seaton B, Head J, Smith K, Flach
CR, Mendelsohn R. 2008. Interaction of recombinant surfactant protein
D with lipopolysaccharide: conformation and orientation of bound
protein by IRRAS and simulations. Biochemistry. 747(31):
8103-8113. doi:10.1021/bi800626h.
Back to Top