Figure 2. Visual representation of Ig Fab proteins bound to
possible AB conformations. (Arndt et al, 2018)
IV. AB Plaque and Mechanism of Degradation
Amyloid-beta accumulation in the brain develops from a
variety of reasons brought on by aging. Interstitial fluid
drainage pathways of the central nervous system can stiffen,
similar to other vascular systems throughout the body. Another
culprit is a transmembrane precursor
protein (APP) which is highly expressed in the brain.
The molecule has a complex degradation pathway and, when
mutated, APP can turn into AB
through proteolytic cleavage.
Amyloid-beta peptides bond together to form AB fibrils
, which make up AB plaque. This causes inflammatory responses in
the brain, as well as neurochemical responses including the
phosphorylation and propagation of the tau
protein. This leads to the formation of tau
aggregates, which cause synaptic loss and the symptoms typically
associated with Alzhiemer's disease.
Unlike previous antibodies, aducanumab exhibits much
more binding with amyloid aggregates rather than monomers or
oligomers, which tend to be more soluble. This allows aducanumab
to target the macromolecules that are actually causing damage to
the brain, rather than binding to the relatively harmless
smaller AB peptides. After crossing the blood-brain barrier (BBB)
and binding to AB plaque, FcY receptors recognize the plaque
coated in aducanumab and signal to microglia cells to erode AB
through phagocytosis.
V. Current Applications and Controversy
Phase I of clinical trials for aducanumab began in 2012.
Both phase I and phase II yielded promising results: the
reduction of amyloid-beta plaque corresponded to an increase in
aducanumab dosage. Phase III of clinical trials began in
September 2015. In these two separate trials, aducanumab (or a
placebo) was administered intravenously once a month in patients
with mild cognitive impairment from Alzhiemer's.
Complications arose when one trial was trending positive (Figure 3)
while the other showed no benefit between the placebo and
aducanumab. Additionally, patients experienced adverse side
effects including nausea (8% of patients), headache (47%),
confusion (15%), cerebral hemorrhage (21%), and cerebral edema
(31%). Cerebral edemas were discovered in patients on high
dosages as well as patients carrying the apolipoprotein E (APOE)
gene. Some sources reason that aducanumab was damaging the BBB
during the receptor-mediated transcytosis, a process needed to
go from the vascular system into the central nervous system.
This led to both trials being stopped prematurely by a
review committee. However, Biogen, aducanumab's manufacturer,
still applied for FDA approval in July of 2020, arguing that
there was sufficient past evidence that showed the drug's
efficacy in higher dosages. The application underwent
accelerated review which included an evaluation by an outside
advisory committee of 11 people. Ten of these individuals voted
against approval, yet their guidance was overruled by FDA
administrators and the drug was approved in June of 2021. Three
members of the committee resigned in protest. Currently,
aducanumab exists as an option for patients at an estimated
$28,200 per year, with the cost varying depending on
dosages.
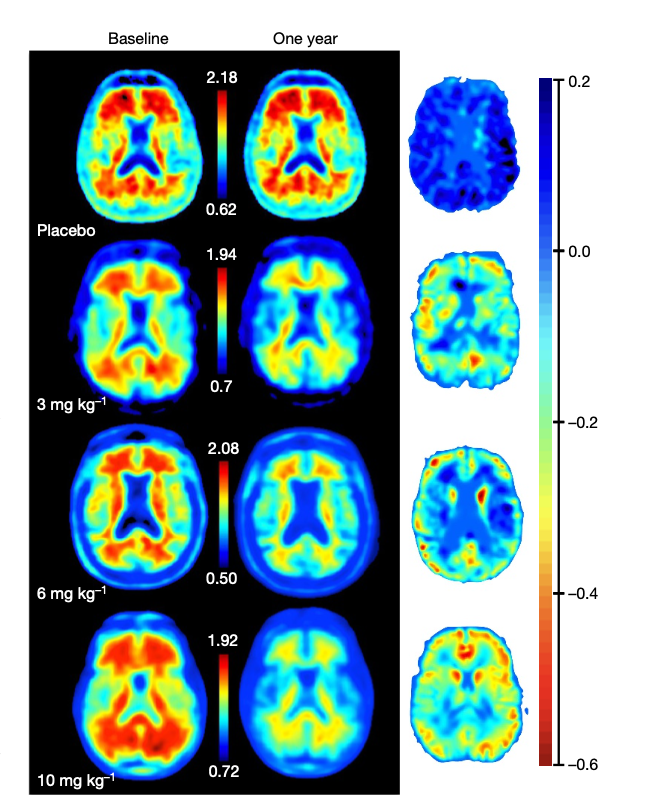
Figure 3. Florbetapir
positron emission tomography of reduced amyloid-beta plaque in
Alzheimer's patients during phase III trials. Amyloid-beta plaque shown in red among patients treated with varying concentrations of aducanumab. (Sevigny et al,
2017)
VI. References
Aducanumab - StatPearls - NCBI Bookshelf
[Internet]. [cited 2022Dec9]. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK573062/
Aducanumab - StatPearls - NCBI
Bookshelf [Internet]. [cited 2022Dec9]. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK573062/.
Aducanumab for the treatment of
alzheimer's disease: Clinical overview ... [Internet]. [cited
2022Dec9]. Available from:
https://www.fda.gov/media/143504/download.
Alzheimer's Association - Wiley Online
Library [Internet]. [cited 2022Dec9]. Available from:
https://alz-journals.onlinelibrary.wiley.com/.
Amyloid beta [Internet]. Amyloid Beta -
an overview | ScienceDirect Topics. [cited 2022Dec9].
Available from:
https://www.sciencedirect.com/topics/medicine-and-dentistry/amyloid-beta.
Amyloid beta [Internet]. Amyloid Beta -
an overview | ScienceDirect Topics. [cited 2022Dec9].
Available from:
https://www.sciencedirect.com/topics/medicine-and-dentistry/amyloid-beta.
Beth Mole - Sep 1 2016 8:08 pm UTC.
Researchers cautiously optimistic about new brain-clearing
alzheimer's drug [Internet]. Ars Technica. 2016 [cited
2022Dec9]. Available from:
https://arstechnica.com/science/2016/09/results-of-small-alzheimers-drug-study-tantalizing-but-no-breakthrough-yet/.
Chen G-fang, Xu T-hai, Yan Y, Zhou
Y-ren, Jiang Y, Melcher K, et al. Amyloid beta: Structure,
biology and structure-based therapeutic development
[Internet]. Nature News. Nature Publishing Group; 2017 [cited
2022Dec9]. Available from:
https://www.nature.com/articles/aps201728.
Ebell MH, Barry HC. Why physicians
should not prescribe aducanumab for alzheimer disease
[Internet]. American Family Physician. 2022 [cited 2022Dec9].
Available from:
https://www.aafp.org/pubs/afp/issues/2022/0400/p353.html#:~:text=With%20regard%20to%20potential%20harms,%2C%20and%20nausea%20(8%25).
Failure to demonstrate efficacy of
aducanumab ... - wiley online library [Internet]. [cited
2022Dec9]. Available from:
https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.12213.
Georgieva JV, Hoekstra D, Zuhorn IS.
Smuggling drugs into the brain: An overview of ligands
targeting transcytosis for drug delivery across the
blood-brain barrier [Internet]. Pharmaceutics. U.S. National
Library of Medicine; 2014 [cited 2022Dec9]. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4279133/.
Immunoglobulin G [Internet]. Wikipedia.
Wikimedia Foundation; 2022 [cited 2022Dec9]. Available from:
https://en.wikipedia.org/wiki/Immunoglobulin_G.
An introduction to antibodies:
Antibody-antigen interaction. [cited 2022Dec9]. Available
from:
https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/protein-biology/elisa/antibody-antigen-interaction.
Jama Network. [cited 2022Dec9].
Available from:
https://jamanetwork.com/journals/jamaneurology/fullarticle/1817720#:~:text=The%20soluble%20building%20blocks%20of,%2Denriched%20microtubule%2Dassociated%20protein.
NHS choices. NHS; [cited 2022Dec9].
Available from:
https://www.nhs.uk/conditions/alzheimers-disease/causes/#:~:text=Alzheimer's%20disease%20is%20thought%20to,form%20tangles%20within%20brain%20cells.
Nimmerjahn F, Ravetch JV. Fc? receptors
as regulators of immune responses [Internet]. Nature News.
Nature Publishing Group; [cited 2022Dec9]. Available from:
https://www.nature.com/articles/nri2206.
O'Brien RJ, Wong PC. Amyloid precursor
protein processing and alzheimer's disease [Internet]. Annual
review of neuroscience. U.S. National Library of Medicine;
2011 [cited 2022Dec9]. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3174086/
Shi M, Chu F, Zhu F, Zhu J. Impact of
anti-amyloid-beta monoclonal antibodies on the pathology and
clinical profile of alzheimer's disease: A focus on aducanumab
and Lecanemab [Internet]. Frontiers. Frontiers; 2022 [cited
2022Dec9]. Available from:
https://www.frontiersin.org/articles/10.3389/fnagi.2022.870517/full#B58.
Tashima T. Brain cancer chemotherapy
through a delivery system across the blood-brain barrier into
the brain based on receptor-mediated transcytosis using
monoclonal antibody conjugates [Internet]. MDPI.
Multidisciplinary Digital Publishing Institute; 2022 [cited
2022Dec9]. Available from:
https://www.mdpi.com/2227-9059/10/7/1597.
Back to Top