Biology Dept Kenyon College |
Immunology |

|
Biology Dept Kenyon College |
Immunology |

|
| Genetics
of
the Immune System
Monoclonal Antibodies Genetics of the Immune System The purpose of the immune system is to attack potential pathogens that have invaded the body. But the pathogens possess defenses, too; they recombine and mutate their own external proteins to thwart the host defenses. Since pathogens reproduce far faster than multicellular hosts, how can host organisms use their own DNA to fight back? The immune system poses
special
problems for genetic regulation:
We shall see how all levels of gene regulation
are involved:
Many classes of cells are involved, including white blood cells and lympocytes. Here we focus on only a tiny fraction of these classes: the B and T lymphocytes which produce antibodies and antigen receptors. Structure of Antibody
Molecule
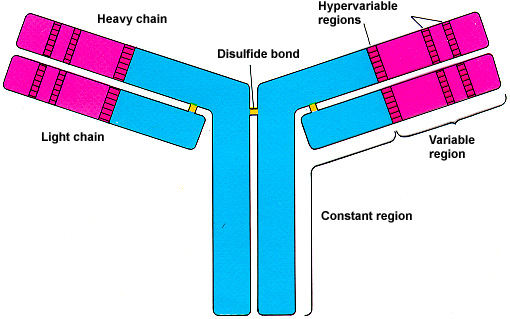 Griffiths et al, AN
INTRODUCTION TO GENETIC
ANALYSIS, 6E, Freeman, 1996
Development of immune cells
To solve the problem of distinguishing self from non-self, the early stages of immune cell development and genetic regulation begins even before birth. DNA rearrangement of antibody genes produces about 107 different clones of pre-antibody producer cells. Then all antibody-producing cells which recognize antigens are eliminated--because any antigen they find must be "self." 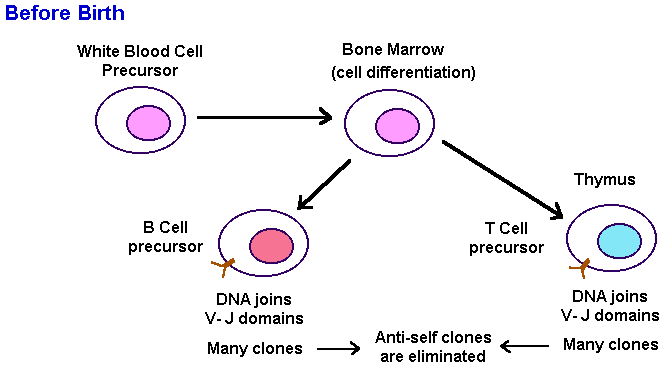 Populations of immune cells recognizing external antigens slowly develop and mature. The maturation of the immune system takes about 18 months after birth--one reason why breast feeding, which provides maternal antibodies, is so helpful during this period. An external antigen stimulates the immune system by binding to the "variable" (i.e. clone-specific) portions of an antigen receptor on a precursor T cell, and on a precursor B cell. Note that antigen receptors are similar to antibodies in structure. The main difference lies in the constant (C) domain. The C domain of antigen receptors keeps them plugged into the membrane, whereas the C domain of antibodies allows secretion.
The B cell then divides, proliferates, and differentiates into:
Where do the ten million different clones come from? The genes encoding antibody and antigen receptors are divided into sets of domains, for portions of a protein with different functions.
Heavy chain: (300 V) (10 D) (4 J) = 12,000
different possible
combinations (VERY approximate)
Note that any one cell will only recombine its DNA in one of these possible ways, and produce one possible antigen receptor or antibody. 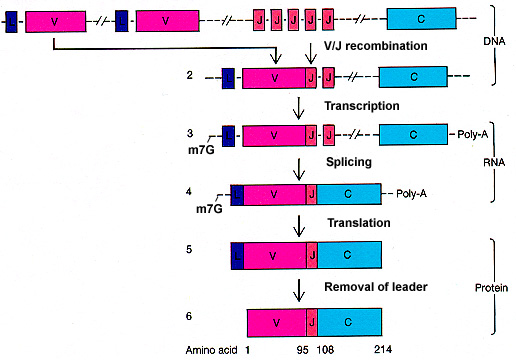 Modified from Griffiths et al,
AN INTRODUCTION
TO GENETIC ANALYSIS, 6E, Freeman, 1996
Expression of the antigen receptor or the
antibody
When the cell has been induced to mature and express its protein, the mRNA has to be spliced:
Where do the two-billion possible antibodies come from? Each time the B and T cell clones are stimulated by an antigen, the clones divide and proliferate. Their sub-clones inherit small mutations in the hypervariable regions (see above). Those sub-clones whose mutations produce slightly stronger-binding antigen receptors will proliferate more than those whose mutations weaken the binding. The product of the possible mutations with the ten-million-odd clones makes billions of possible antibody structures! Monoclonal Antibody Technology Modified from the MIT Hypertextbook, Excerpted from "What is Biotechnology?" Washington, D.C.: Biotechnology Industry Organization, 1989. Obtained from Genentech's Access Excellence From the standpoint of gene technology, antibody molecules have two very useful characteristics:
The specificity of antibodies makes monoclonal antibody technology so valuable for biotechnology. Not only can antibodies be used therapeutically, to protect against disease; they can also help to diagnose a wide variety of illnesses, and can detect the presence of drugs, viral and bacterial products, and other unusual or abnormal substances in the blood. Given such a diversity of uses for these disease-fighting substances, their production in pure quantities has long been the focus of scientific investigation. The conventional method was to inject a laboratory animal with an antigen and then, after antibodies had been formed, collect those antibodies from the blood serum (antibody-containing blood serum is called antiserum). There are two problems with conventional antibodies:
These antibodies are called monoclonal because they come from only one type of cell, the hybridoma cell; antibodies produced by conventional methods, on the other hand, are derived from preparations containing many kinds of cells, and hence are called polyclonal. An example of how monoclonal antibodies are derived is described below. 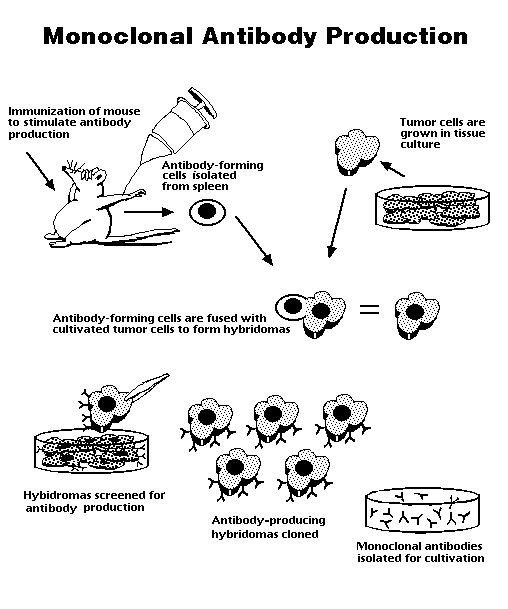 A myeloma is a tumor of the bone marrow that can be adapted to grow permanently in cell culture. Myeloma cells can be fused with antibody-producing mammalian spleen cells, using a membrane fusion agent such as polyethylene glycol (PEG) or SV40 virus. The nuclei fuse, and their chromosomes replicate and undergo mitosis together. The resulting hybrid cells, or hybridomas, produce large amounts of monoclonal antibody. This product of cell fusion combines the desired qualities of the two different types of cells: the ability to grow continually, and the ability to produce large amounts of pure antibody. Monoclonal antibodies have enormous clinical as well as
research
applications; for example, the Western Blot test
for HIV.
|