Biology Dept Kenyon College |
|

|
Biology Dept Kenyon College |
|

|
| Plant
development: flowers
Drosophila development Animal development: fertilization and cleavage Flower organs form in concentric rings, called whorls, starting with the outer whorl. Sepals form first, followed by petals, and then by stamen, and finally by carpels. Below is an image of a wild type Arabidopsis thaliana flower, and a diagram of the flower outlining the four whorls. Wild type Arabidopsis flowers have, from outside to inside, 4 sepals (which are not clearly visible in the photograph), 4 petals, 6 stamens, and 2 carpels which fuse to form the central pistil.
How do the proper flower parts form in the proper arrangment? Members of Elliot Meyerowitz's and Enrico Coen's laboratories have used genetic dissection to identify genes required for proper floral organ identity in Arabidopsis thaliana and in Antirrhinum majus(snapdragon). These scientists identified homeotic mutations that resulted in the misplacement of floral organs. Three classes of mutations were identified in Arabidopsis. Class A: flowers with these mutations have (unfused) carpels instead of sepals in whorl 1, and stamens instead of petals in whorl 2. The pattern of organs (from outside to inside) is carpel, stamen, stamen, carpel. The genes containing these mutations were named APETALA1 (AP1) and APETALA2 (ap2). Below is an image of an apetala2 mutant flower (right) next to a wild type flower (left).
Class B: flowers with these mutations have sepals in whorl 2 instead of petals, and (unfused) carpels in whorl 3 instead of stamens. The pattern of organs (from outside to inside) is sepal, sepal, carpel, carpel. The genes containing these mutations were named APETALA3 (AP3) and PISTILLATA (PI). Below is an image of a pistillata mutant flower (right) next to a wild type flower (left).
Class C: flowers with this mutation have petals in whorl 3 instead of stamens, and sepals in whorl 4 instead of carpels. In addition, the floral meristem is not determinate - flowers continue to form within the flowers, so the pattern of organs (from outside to inside) is sepal, petal, petal; sepal, petal, petal; sepal, petal, petal, etc. The gene containing this mutation was called AGAMOUS (AG). Below is an image of an agamous mutant flower (right) next to a wild type flower (left).
The ABC model: Each class of genes is required in two adjacent whorls. Class A genes are required in whorls 1 and 2, class B genes are required in whorls 2 and 3, and class C genes are required in whorls 3 and 4. Both class A and class B genes are required in whorl 2, and both class B and class C genes are required in whorl 3. The ABC model that summarizes these results is shown below. We'll talk in class about how this model has been tested.
The Drosophila life cycle consists of a number of stages: embryogenesis, three larval stages, a pupal stage, and (finally) the adult stage!
Embryogenesis
in Drosophila
from LIFE: The Science of Biology, Purves et al, 1998
Genetic
Analysis of Drosophila development
Nusslein-Volhard
and Wieschaus
set out to identify EVERY GENE required for early pattern formation in
the Drosophila embryo. They looked for recessive embryonic lethal
mutations,
and classified them according to their phenotype before death. That is,
they looked for and analyzed dead embryos. Images of some of the
mutants
they identified are shown below. Notice the differences in segmentation
patterns between the wildtype, shown on the left, and the mutant
embryos.
An online historical essay about their work is here.
images from the October 30, 1980 issue of Nature A cascade of gene activation sets up the Drosophila body plan The maternal-effect genes, including bicoid and nanos, are required during oogenesis. The transcripts or protein products of these genes are found in the egg at fertilization, and form morphogen gradients. The maternal-effect genes encode transcription factors that regulate the expression of the gap genes. The gap genes roughly subdivide the embryo along the anterior/posterior axis. The gap genes encode transcription factors that regulate the expression of the pair-rule genes. The pair-rule genes divide the embryo into pairs of segments. The pair-rule genes encode transcription factors that regulate the expression of the segment polarity genes. The segment polarity genes set the anterior/posterior axis of each segment. The gap genes, pair-rule genes, and segment polarity genes are together called the segmentation genes, because they are involved in segment patterning.
from LIFE: The Science of Biology, Purves et al, 1998 But how do these segments take of individual identities? In normal flies,
structures
like legs, wings, and antennae develop on particular segments, and this
process requires the action of homeotic genes. Enter Ed Lewis,
who
discovered homeotic mutants - mutant flies in which structures
characteristic
of one part of the embryo are found at some other location.
The homeotic
genes encode
transcription factors that control the expression of genes responsible
for particular anatomical structures, such as wings, legs, and
antennae.
The homeotic genes include a 180 nucleotide sequence called the homeobox,
which is translated into a 60 amino acid domain, called the
homeodomain.
The homeodomain is involved in DNA binding, as shown in the images
below.
Homeobox-containing (or HOX) genes are found in many organisms, including worms, fish, frogs, birds, mammals, and plants. Interestingly, HOX genes are found in clusters, and the relative gene order within these clusters in conserved between organisms. That is, the order of related HOX genes in Drosophila and in mice is the same! In addition, the order of HOX-genes on the chromosome is related to where they are expressed along the anterior/posterior axis.
from LIFE: The Science of Biology, Purves et al, 1998 Animal development: fertilization The main function of fertilization is to combine the haploid sets of chromosomes from two individuals into a single diploid cell, the zygote. In addition, fertilization activates the egg. Egg activation blocks entry by additional sperm, stimulates the final meiotic division, and triggers the onset of embryonic development. Sperm entry: The acrosomal
reaction
is a change in the sperm that is common to many higher animals. In the
sea urchin, receptor proteins
(red)
in the sperm plasma membrane contact the sea urchin jelly coat, causing
Na+ channels in the plasma membrane to open. Na+ ions flow into the
sperm
head and the plasma membrane depolarizes, opening voltage-sensitive
Ca++
channels. Ca++ ions flow into the sperm head, and the high Ca++ causes
the fusion of the acrosomal vesicle
(green) with the sperm plasma membrane, releasing acrosomal vesicle
contents
into the extracellular space. Digestive enzymes from the acrosomal
vesicle
digest the jelly coat and vitelline membrane. Ca++ also activates a
Na+:H+
ion exchanger, which pumps H+ out of the cell, increasing intracellular
pH. This pH change causes the polymerization of actin
subunits (pink) into microfilament cables that thrust
acrosomal
processes toward the egg plasma membrane. Bindin
(blue) released from the acrosomal vesicle coats the acrosomal process.
The increase in intracellular [ion] causes water to enter the cell,
increasing
hydrostatic pressure. This aids in the extension of the acrosomal
process.
After making its
way through
the jelly coat, the sperm makes contact with the vitelline envelope.
Species-specific
bindin receptors on the vitelline envelope are only able to recognize
bindin
molecules from the same species. This "lock and key" mechanism
ensures
that eggs are fertilized only by sperm of the same species. After
making
its way through the vitteline envelope, the sperm and egg plasma
membranes
fuse, and the sperm nucleus enters the cytoplasm of the egg.
Preventing polyspermy: Although many sperm attach to the coats surrounding the egg, it is important that only one sperm fuses with the egg plasma membrane and delivers its nucleus into the egg. Two mechanisms are used by animals to ensure that only one sperm fertilizes a given egg: the fast block to polyspermy and the slow block to polyspermy. Fast block to
polyspermy:
Slow block to polyspermy: The slow block to polyspermy begins within 10 seconds of fusion of the sperm and egg plasma membranes. A compound called inositol triphosphate (IP3) causes the release of Ca++ from intracellular stores in the egg endoplasmic reticulum. Ca++ is first released at the site of sperm entry, and during the next minute, a wave of free Ca++ passes through the egg. This Ca++ results in the fusion of cortical vesicles with the egg plasma membrane, releasing their contents into the space surrounding the egg, called the perivitelline space. This raises the vitelline membrane, and inactivates bindin receptors on the vitelline membrane. Thus, any additional sperm are released from the vitelline membrane and no more bind. 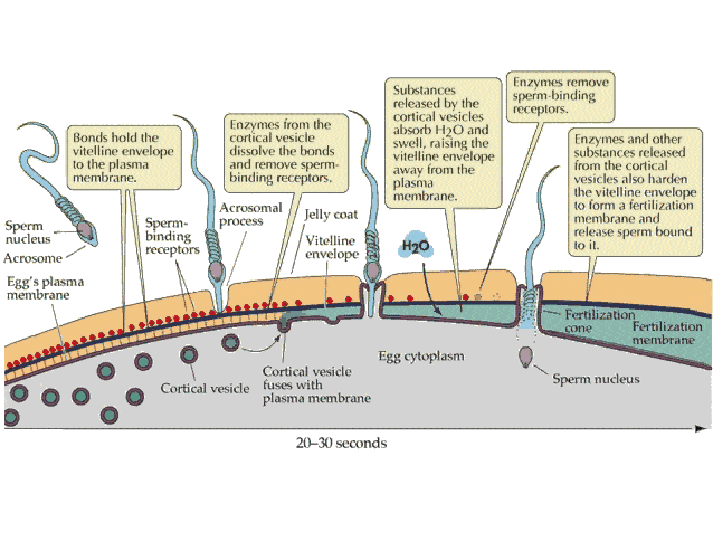 from LIFE: The Science of Biology, Purves et al, 1998 Click on the image below to watch a movie showing the events involved in the slow block to polyspermy! This movie shows simultaneous double imaging of phase contrast (left) and intracellular Ca ++ concentration (right) during sea urchin fertilization. The left image shows the approach of the sperm at about 2 o'clock and the rising of the vitelline membrane. Intracellular Ca++ is monitored by an indicator that becomes more fluorescent when it binds Ca++. A transient Ca++ rise around the entire cortex (fifth frame) is followed by the Ca++ wave, which begins at the sperm entry site. 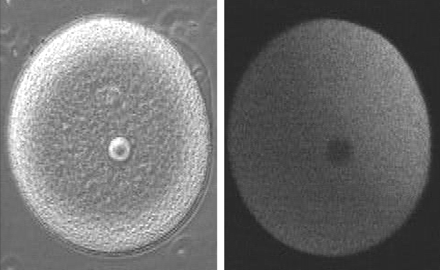
image and movie from Mark Terasaki
Egg activation Ca++ release at fertilization results in an increase in metabolic activity within the egg, apparently due to an increase in the intracellular pH of the egg. Diacyl gycerol (DAG) causes protein phosphorylation cascades to be initiated, with one result being the phosphorylation and activation of a plasma membrane Na+:H+ ion exchanger. Na+ is pumped into the cell, H+ is pumped out of the cell, and the pH inside the cell increases. Sperm themselves are NOT required for egg activation - injection of Ca++ can artificially induce egg activation in many species. Cortical rotation Positional information is already contained within many eggs, with the exception of mammals. Egg polarity is due to the asymmetric distribution of cytoplasmic molecules, including mRNAs, proteins, and yolk, and is roughly oriented along the anterior/posterior axis in most animals. A rearrangment of the egg cytoplasm is induced by fertilization within many frog species, and this rearrangment (called cortical rotation) results in the establishment of the dorsal/ventral axis. During cortical rotation, the plasma membrane and cortex (cytoplasmic region just below the plasma membrane) rotate relative to the inner cytoplasm. The pigmentation of frog eggs makes it possible to observe this process visually, and results in a gray area, called the gray crescent.
Cortical Rotation Helps Establish Positional Information by Affecting the Distribution of Cytoplasmic Determinants like beta-catenin. Click here for details. Cleavage is a series of rapid cell divisions without cell growth or gene expression which occurs in early embryogenesis. Early cleavage divisions in most embryos are reductive. During cleavage, the cytoplasm is divided into smaller and smaller cells, called blastomeres. The total cellular volume of the embryo stays the same, but the number of cells within the embryo increases. Cleaving cells have a modified cell cycle, in which the two gap phases, G1 and G2, are completely omitted. The cells cycle rapidly between M and S phases.
from the Amphibian Embryology Tutorial Cleavage results in a blastula, a ball of cells with a central cavity called the blastocoel. Below are two diagrams of blastulas. On the left is a sea urchin blastula, and on the right is a frog blastula.
The pattern of cleavage is influenced by the amount of yolk in the egg. In eggs with less yolk, cleavages are equal, and the resulting blastomeres are of similar size (a). If the yolk is localized, such as in frog eggs, then cleavages are unequal - the cells derived from the yolky region (the vegetal pole) are larger than those derived from the region without yolk (the animal pole) (b). 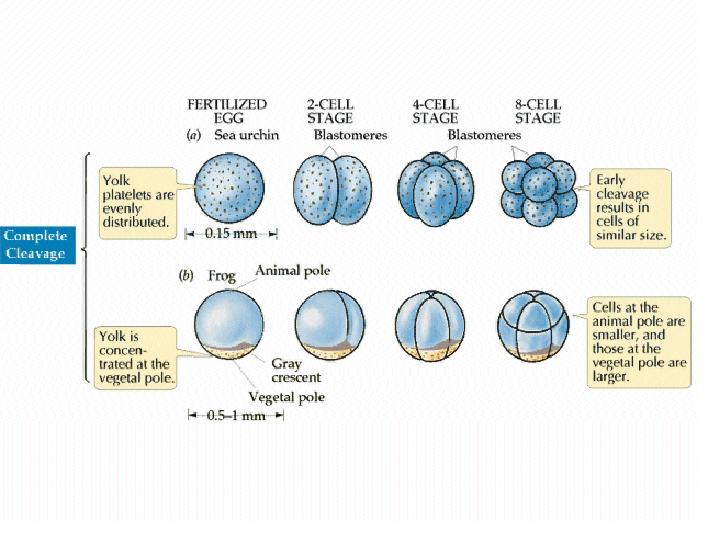 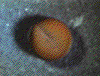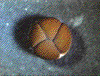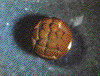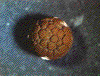 (No movement? Click reload/refresh)
(No movement? Click reload/refresh)
from Bill Wasserman's Developmental Biology Page
In the very yolky eggs of fish and birds, the cleavage furrow is slowed, or even blocked, by the presence of the yolk. Complete divisions are restricted to the least yolky region of the egg, and the embryo forms as a cap of cells sitting on top of the yolk (c). See images of zebrafish cleavage below. 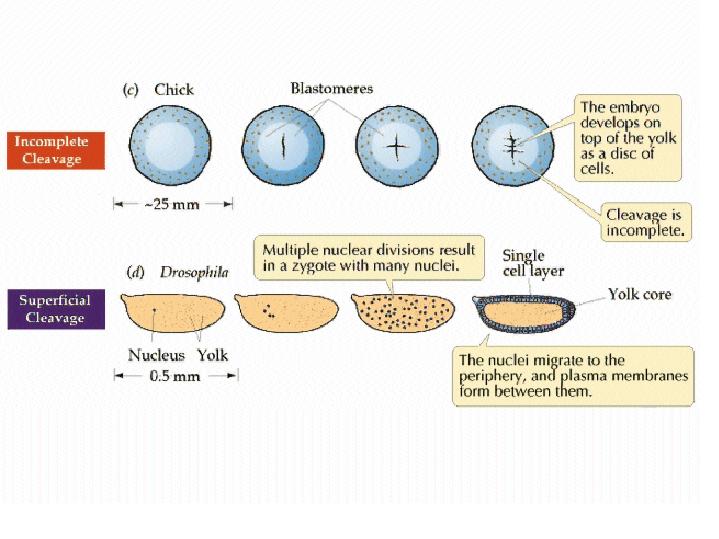 from Purves et al, LIFE: the Science of Biology Cleavage in the zebrafish embryo is limited to a small region on "top" of the yolky mass.
Early cleavage patterns vary widely between different groups of animals, based largely on the orientation of the division planes. The simplest pattern is radial cleavage, in which successful division planes are at 90 degree angles relative to each other. This results in the blastomeres aligned directly over or to the side of one another. In spiral cleavage, the division planes are not at 90 degree angles, resulting in blastomeres that are NOT aligned directly over or beside one another. Click on the image below to get a better sense for the difference between these two cleavage patterns in two generalized embryos.
Radial cleavage in the sea urchin results in a hollow sphere of cells.
The mammalian zygote gives rise to both the embryo and extraembryonic tissues, such as the placenta. Changes in cell behavior and cell cleavage patterns during early embrogenesis results in a 32-cell blastocyst consisting of the inner cell mass, which will form the embryo, and the trophoblast, which will form extraembryonic tissues.
Watch this animation of human development from University of Pennsylvania Health System Basic Embryology Review Program. Focus on the inital stages of development, up until implantation. Images of human embryos are available from the Multi-dimensional Human Embryo and from the Visible Embryo Thanks to David Marcey for some of the images and text shown above << Return to Syllabus << |