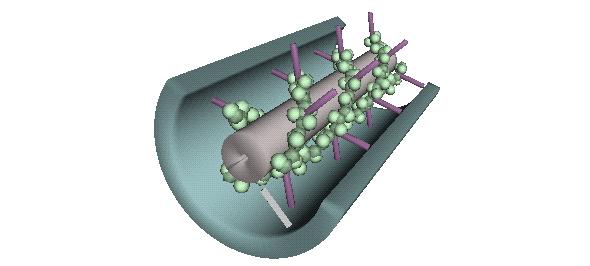


Signaling and Transport Mechanisms of Plasmodesmata
Other Functions of Plasmodesmata


--figure from Micrograph of the Month at Brown University, Dr. Kenneth R. Miller
Ever since the electron microscope was introduced into the battery of scientific tools available to researchers, plasmodesmal studies have become an exciting field in plant physiology. When previously it was thought that these pores were merely passive tunnels for small solute transport between cells, there are now fervent debates about the structure, composition, and active transport dynamics of these intercellular channels.
Composition and Structure
Since the function of plasmodesmata are so closely associated with their rather complex structure, it is necessary to delinate the make-up of plasmodesmata before we discuss their functions in plants. It has been demonstrated that the plasma membrane is continuous between cells, the outer leaflet contiguous with the cell wall and the inner leaflet contiguous with the plasmodesmal pore (general structure reviewed by Epel, 1994; Overall and Blackman, 1996; and Oparka, 1993). Within the plasmodesmal pore, a tightly wound cylinder of membrane termed the desmotubule runs the length of the plasmodesma. Thus, the desmotubule is essentially a tube within a larger tube bordered by the plasma membrane. The structure of the desmotubule and how it relates to the overall structure of plasmodesmata was studied by Tilney, et al. (1991) by using plasmolysis, Triton X detergent extraction, and protease digestion. This investigation utilized fern (Onoclea sensibilis) gametophytes by cutting them in half, exposing the cut surfaces to Triton X 100, and then fixing the gametophytes. This detergent solubilized the plasma membrane component of the plasmodesmata of the gametophytes, but the desmotubule was not affected by the detergent. However, when the cut gametophyte surfaces were exposed to papain, the desmotubule is destroyed but the plasma membrane remained intact (yet swollen). Finally, the gametophytes were plasmolyzed, and it was found that plasmodesmata remained intact as long as the desmotubule stayed in its normal, fixed position as the cells detached from the cell walls. Thus, Tilney, et al. (1991) suggested that the desmotubule provides a rigid stability to plasmodesmata and confers a fixed diameter and pore size to the plasmodesmal canal, much like a cytoskeletal structure. However, one should be cautious in mistaking the desmotubule as a completely rigid strcuture, since the desmotubule is linked to the endoplasmic reticulum in each of the adjacent cells, forming a dynamic endomembrane continuum in the symplastic space, a topic to be discussed in more detail later (Grabski, et al., 1993).
The space between the plasmalemma and the desmotubule is the cytoplasmic sleeve or cytoplasmic annulus, and transport through plasmodesmata has been proposed to occur either through the lipid portions of the desmotubule or this cytoplasmic sleeve (or both):
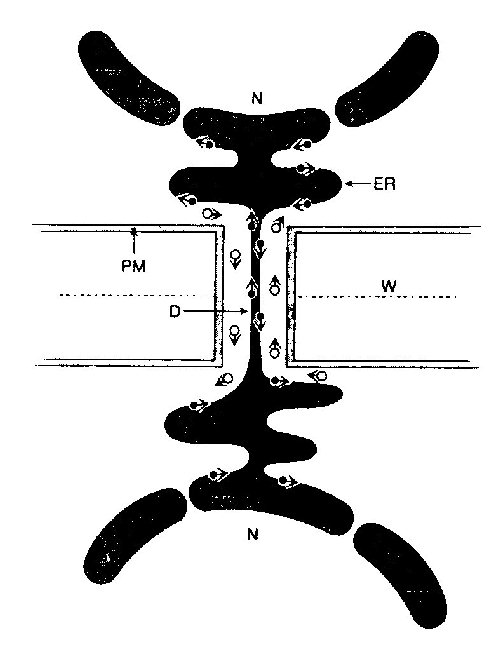
--figure from Oparka, 1993
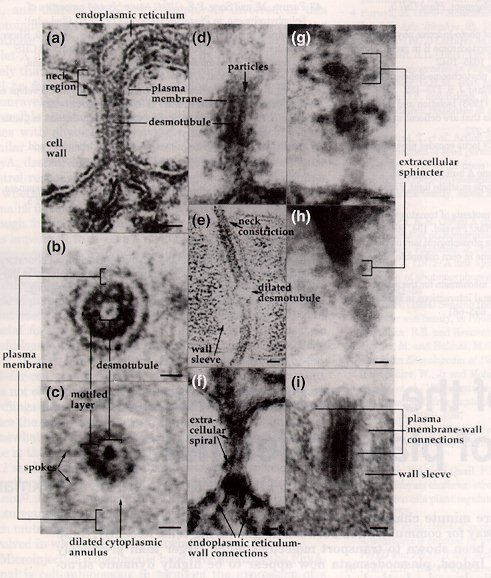
In some plasmodesmata, there is a region at each end of the plasmodesmal channel that is constricted and termed the neck region, where the plasma membrane component of the plasmodesmata closely associates with the central desmotubule. The neck region is proposed to contain several, spoke-like protein subunits that are located both extracellularly and between the desmotubule and the plasmalemma (linking these two structures), and these proteins can then act as a sphincter to regulate the passage of materials through the plasmodesmata, much like gap junctions in animal cells (reviewed by Robards and Lucas, 1990):
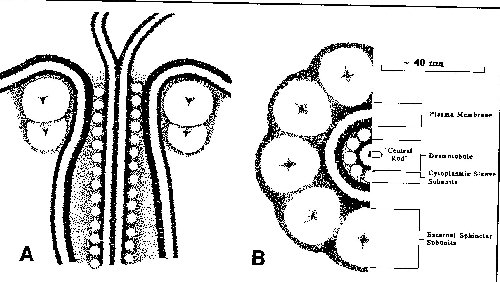
--figure from Robards and Lucas, 1990
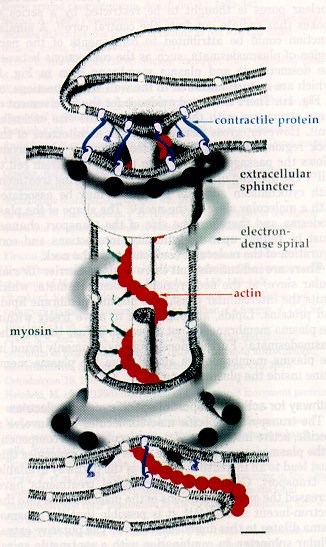
Recently, studies by White, et al. (1994) have suggested that there is an actin-myosin network associated with plasmodesmata, since these proteins were found to be associated with plasmodesmata using immunogold cytochemistry. Overall and Blackman (1996) suggest that the actin and myosin wrap around the central desmotubule and help to stabilize the desmotubule and consequently the entire plasmodesma. They support their claim by providing evidence that treatment of plant tissue with cytochalasin (which interferes with actin structures) have cells with plasmodesmata that are swollen, much like the plasmodesmata in the gametophyte tissue exposed to papain in the investigation by Tilney, et al. (1991). The possible roles of actin and myosin in plamodesmal function are discussed below. Here is the most recent diagramatic model of plasmodesmal structure, proposed by Overall and Blackman (1996):
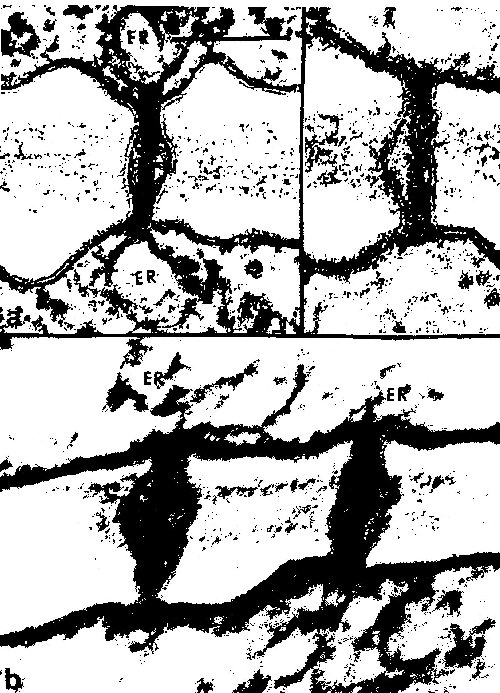
Electron microscopy has been an essential tool for discerning plasmodesmata structure, but one should be aware that the dynamics of plasmodesmal transport should not and cannot be absolutely inferred from these static images. But, these images are still the major source of information on the substructure of plasmodesmata; here are some examples of electron micrographs of plasmodesmata (figures from Overall and Blackman, 1996; Tilney, et al., 1991; and Hepler, 1982, respectively):

Computer-generated models of plasmodesmata structure have also been useful for describing the three-dimensional characteristics of the plasmodesmal canal. Below are computer-generated images of various models described in the literature (images from Chris Betts at Monash Univ.):

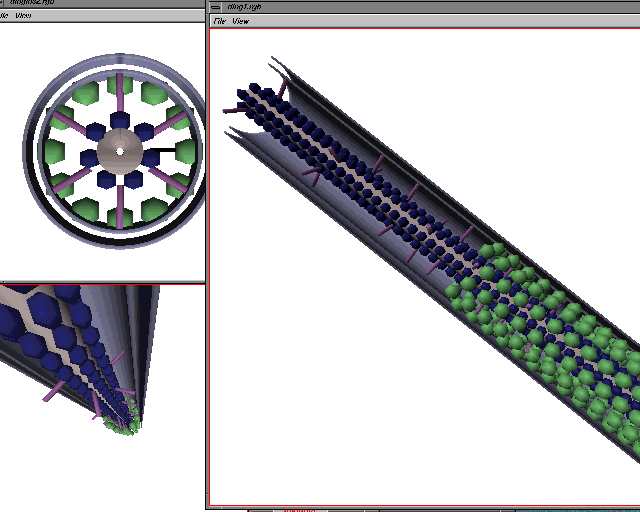

THE OVERALL MODEL
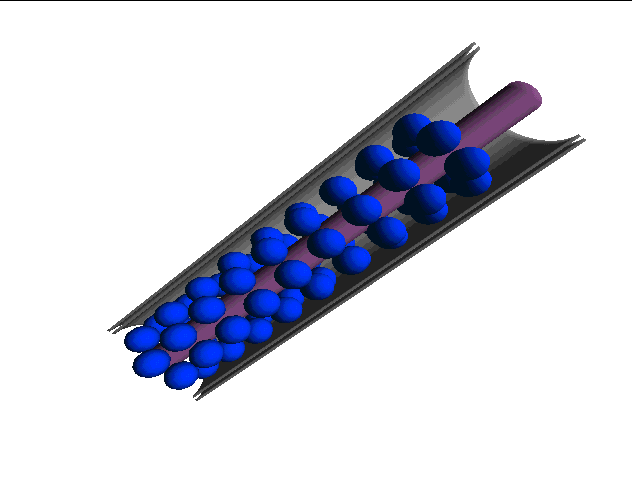

THE RADFORD MODEL
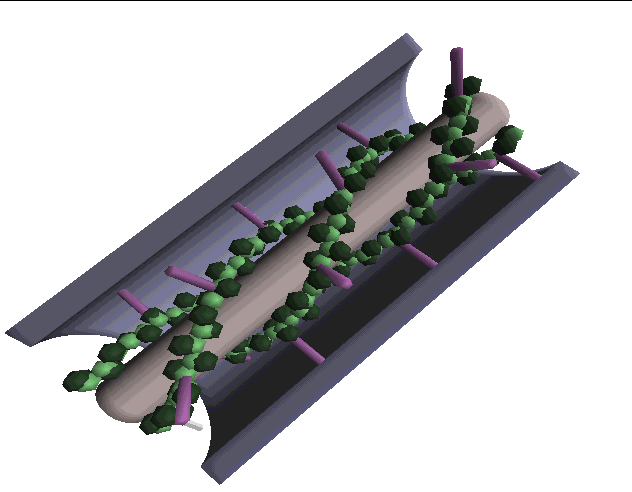

THE WHITE MODEL


A more recent study by Grabski, et al. (1993) provided a detailed model of lipid diffusion and transport between contiguous soybean root cells via the endoplamsmic reticulum. This investigation utilized the experimental technique of fluorescence redistribution after photobleaching cells to determine the mobility of various fluorescently-labeled lipids and phospholipids, many of which are important signalling molecules in plant cells. Some of the lipid molecules used in this study predominantly resided in the plasma membrane while the others primarily were mobile through the endoplasmic reticulum only (determined by confocal microscopy). When these various lipids were introduced to the soybean cells, only those molecules associated with the endoplasmic reticulum were transported from cell to cell via plasmodesmata, whereas the lipids in the plasma membrane did not cross intercellularly. A particularly noteworthy observation was that, when 1-acyl-2-(N-4-nitrobenzo-2-oxa-1,3-diazole)aminoacylphosphatidylcholine was introduced as one of the reporter molecules in the plasma membrane, it was quickly converted to 1-acyl-2-(N-4-nitrobenzo-2-oxa-1,3-diazole)aminoacyldiglyceride (NBD-DAG), which is then translocated to the endoplasmic reticulum. The newly-formed NBD-DAG, an analog of the second messenger DAG of the phosphatidylinositol signaling pathway (reviewed by Grabski, et al., 1993) could then be transported between cells, which has great functional importance for intercellular communication and transport of signalling molecules. Overall, this study by Grabski, et al. (1993) offered two important ideas about a dynamic membrane continuum between plant cells:
Why do the parameters of passive diffusion through plasmodesmata vary so much among investigations? One explanation is that the microinjection technique used to exogenously add small probe molecules may be triggering a wound response in the plant cell and a consequent partial closure of plasmodesmata, perhaps by callose plugging up the transport pathways (Overall and Blackman, 1996). The varying size exclusion limits may also be due to the specific spoke molecules allowing passive transport through the neck region of plasmodesmata; different species of plants may have different neck region compositions, accounting for the discrepancies in size exclusion limits for passive transport (Epel, 1994).
Grabski, S, de Feijter, AW, and Schindler, M. (1993) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. The Plant Cell 5:25-38.
Hepler, PK. (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111:121-133.
Oparka, KJ. (1993) Signalling via plasmodesmata--the neglected pathway. Semin. Cell Bio. 4: 131-138.
Overall, R and Blackman, L. (1996) A model of the macromolecular structure of plasmodesmata. Trends in Plant Science 1 (9):307-311.
Robards, AW, and Lucas, WJ. (1990) Plasmodesmata. Annu Rev Plant Physiol Plant Mol Bio 41:369-419.
Tilney, LG, Cooke, TJ, Connelly, PS, and Tilney MS. (1991) The structure of plasmodesmata as revealed by plasmolysis, detergent extraction, and protease digestion. The Journal of Cell Biology 112 (4):739-747.
Wang, N, and Fisher, DB. (1994) The use of fluorescent tracers to characterize the post-phloem transport pathway in maternal tissues of developing wheat grains. Plant Physiol 104:17-27.
White, RG. (1994) Actin associated with plasmodesmata. Protoplasma 180:169-184.
Xiong, Z (1996) Cell-to-cell and Long Distance Movement of Plant Viruses, lecture notes. http://ag.arizona.edu/~zxiong/plp611/lect11.html
Zambryski, P (1995) Plasmodesmata: Plant Channels for Molecules on the Move. Science 270, http://www.sciencemag.org/science/scripts/display/full/270/5244/1943.html
A dynamic endomembrane continuum
During cytokinesis of plant cells, the endoplasmic reticulum has been associated with the dictyosomal vesicles of the forming phragmoplast of the dividing cell (reviewed by Hepler, 1982). Sometimes, portions of the endoplasmic reticulum are trapped within these vesicles as they fuse, these tubular ER elements manage to traverse the cell plate, and plasmodesmata are formed. A study by Hepler (1982) substantiated the model that the desmotubule was a tightly furled stretch of endoplasmic reticulum that confers a membranous continuum between adjacent cells. Previous methods for endoplasmic reticulum staining had proved difficult: potassium permanganate (KMnO4) staining managed to stain the ER heavily but did not provide enough structural detail (because it degraded various cellular structures), and glutaraldehyde-OsO4 fixation was gentle enough to not disrupt cytoplasmic details but did not stain heavily enough for proper contrast of ER membrane (Hepler, 1982). However, this study by Hepler (1982) used a novel staining technique that utilized a mixture of osmium tetroxide and potassium ferricyanide to selectively and strongly stain for ER without destroying any cellular structures. Through this staining technique, Hepler (1982) was able to demonstrate that the endoplasmic reticulum did, in fact, run throughout the plasmodesmata of contiguous lettuce (Lactuca sativa) tissue cells, and the cisternal space of the endoplasmic reticulum was occluded by the tightly-constricted neck regions of the plasmodesmata (see electron micrograph # , above), providing support for the notion that the desmotubule was a strong, tightly-wound rod running down the length of plasmodesmata.
Signaling and Transport Mechanisms of Plasmodesmata
Classical studies on transport through plasmodesmata have utilized microinjection of small, fluorescently-labeled probes to examine the passive transport mechanisms of plasmodesmata. These probes were first used to describe the size-exclusion limits, or the maximum size of a molecule that can pass through plasmodesmata passively, of plasmodesmata in various plant species. Looking at the cytoplasmic sleeve region and the spokes that constrict the neck region plasmodesmata, it was determined that the average width of channels allowing molecules to passively travel through a plasmodesmal annulus is approximately 3 nm, and thus the average size exclusion limit is molecules weighing approximately 800-1000 Da (reviewed by Oparka, 1993; Epel, 1994; Robards and Lucas, 1990; and Overall and Blackman, 1996).Variations on passive transport
However, it has recently been shown that size exclusion limits vary between species and even cell types during passive transport. In tobacco mesophyll cells, the size exclusion limit of fluorescently-labeled dextrans is only 1 kDa, whereas in trichome cells, the size-exclusion limit is as large as 7 kDa (reviewed by Overall and Blackman, 1996 and Epel, 1994). Another study by Wang and Fisher (1994) employed normally apoplastic probes such as Lucifer Yellow and incubated sections of crease tissue from developing wheat grains in solutions of these probes. The dye moved into the symplastic space through plasmodesmata, and it was determined that in all cells except the pericarp, the diameter of the plasmodesmata in this tissue was 6.2 nm (twice the normal exclusion limit).Active transport of macromolecules
Other macromolecules destined for intercellular transport are shuttled through plasmodesmata via active transport mechanisms. Some studies have shown that plasmodesmata can alter their dimensions such that they expand/dilate outward into an electron-lucent sleeve surrounding the normal-sized plasmodesmata (reviewed by Overall and Blackman, 1996). This expansion would allow for the transport of larger molecules through the cytoplasmic sleeve. The mechanism or trigger of this plasmodesmal dilation is virtually unknown, but it has been recently postulated that proteins in the neck region may be implicated in this phenomenon (Overall and Blackman, 1996).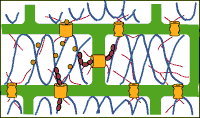 Another major recent finding concerning active transport through plasmodesmata involves a possible association of cytoskeletal elements with both trafficking/targeting molecules to the plasmodesmata and assisting in the energetics of active transport. It has been demonstrated that the endoplasmic reticulum is closely associated with microtubules, and viral movement proteins (discussed below) have been shown to track through the plant cell along these microtubules (reviewed by Overall and Blackman, 1996). Actin and tubulin have both been shown to bind to viral movement protein, which transports itself through the plant via plasmodesmata (reviewed by Zambryski, 1995). Thus, cytoskeletal elements are helping to guide various macromolecules to plasmodesmata, and also actin has been thought to help loosen the sphincter elements of the neck region to allow for the transport of these larger molecules (reviewed by Overall and Blackman, 1996). In addition, myosin may also act as a cytoskeletal motor to provide the energetics for active plasmodesmal transport. Myosin has been found in several species of plants and is an ATP-dependent protein that generates directional movement (as in flagellar motion) (Overall and Blackman, 1996). High ATPase activity has been demonstrated in plasmodesmata, so myosin is a prime candidate for aiding active transport and may be the spokes, sometimes seen in electron micrographs, connecting the desmotubule to the cytoplasmic annulus throughout the plasmodesmata(Overall and Blackman, 1996).
Another major recent finding concerning active transport through plasmodesmata involves a possible association of cytoskeletal elements with both trafficking/targeting molecules to the plasmodesmata and assisting in the energetics of active transport. It has been demonstrated that the endoplasmic reticulum is closely associated with microtubules, and viral movement proteins (discussed below) have been shown to track through the plant cell along these microtubules (reviewed by Overall and Blackman, 1996). Actin and tubulin have both been shown to bind to viral movement protein, which transports itself through the plant via plasmodesmata (reviewed by Zambryski, 1995). Thus, cytoskeletal elements are helping to guide various macromolecules to plasmodesmata, and also actin has been thought to help loosen the sphincter elements of the neck region to allow for the transport of these larger molecules (reviewed by Overall and Blackman, 1996). In addition, myosin may also act as a cytoskeletal motor to provide the energetics for active plasmodesmal transport. Myosin has been found in several species of plants and is an ATP-dependent protein that generates directional movement (as in flagellar motion) (Overall and Blackman, 1996). High ATPase activity has been demonstrated in plasmodesmata, so myosin is a prime candidate for aiding active transport and may be the spokes, sometimes seen in electron micrographs, connecting the desmotubule to the cytoplasmic annulus throughout the plasmodesmata(Overall and Blackman, 1996).Viruses and Plasmodesmata
A special subset of active plasmodesmal transport involves the intercellular movement of plant viruses through plasmodesmata. Most, if not all, plant viruses utilize plasmodesmata to travel around and infect an entire plant (Epel, 1994), and there is strong evidence to suggest that the viruses manipulate the plasmodesmata to allow large viral particles (several times larger than the normal size exclusion limt) to pass between cells. The major mechanism of this type of viral transport has been deduced through studies of the tobacco mosaic virus, which encodes for a movement protein (MP) that mediates the transport of a non-virion form of the virus between cells (Epel, 1994). The mechanism is thought to be as follows (adapted from Xiong, 1996):
Three functional domains have been located in the viral movement protein: one for RNA binding, one for cooperative RNA binding, and one for plasmodesmal interaction (Xiong, 1996). The exact mechanism for any of the above steps is not yet known, but it has been demonstrated that the viral movement protein is acting on plasmodesmata directly to increase their size exclusion limits, since fluorescently-labeled large dextrans (between 9-35 kDa) have been found to move between cells in transgenic plants expressing the movement protein, and no endogenous metabolism of the dextrans was detected (Xiong, 1996). Viral movement protein studies will continue to allow researchers to more clearly define the mechanisms of plasmodesmal targeting and transport.Other Functions of Plasmodesmata
Plasmodesmata have also been shown to play a role in numerous other plant cell processes (reviewed by Oparka, 1993):
In conclusion, plasmodesmata play a crucial role in transporting materials and signalling molecules intercellularly in higher plants. In the last decade, it has been discovered that plasmodesmal function is much more complex that previously imagined, and more molecular and in vivo studies are necessary to discern the absolute structure and function of these interesting cytoplasmic channels.REFERENCES CITED
 Brought to you by Hannahs, Inc., productions
Brought to you by Hannahs, Inc., productions