Vesicular Arbuscular Mycorrhizae
"...The mycorrhizal condition is the normal state for most plants under most ecological conditions."
Mycorrhizal Symbiosis, Smith and Read 1997
Vesicular arbuscular mycorrhizae (VA mycorrhizae, or VAM) are the most abundant of a group of symbiotic fungi that infect plant roots. VA mycorrhizae obtain carbon from their
plant hosts, and (usually -- any generalization about VA mycorrhizae is risky) increase the nutrient and water uptake of their hosts. Some species of VA mycorrhizae colonize only
particular plant hosts, while others are less specific. VA mycorrhizae consist of both external and intraradical structures, with the most distinctive
structures occurring inside the root. A description of VA mycorrhizal physiology, though, is perhaps better started with the external mycorrhizal structures.
The most visible VA mycorrhizal structure is the hyphal network. Hyphae are thin (from 2 µm in diameter to > 20 µm) hollow tubes of fungi, which in VA mycorrhizae have few
cross walls (are aseptate) and have distinct angular projections (Sylvia in Norris et al. 1994). These tubes originally grow from fungal spores, extending short distances (on the order
of millimeters) into the soil in search of the roots of host plants (Smith and Read 1997). If the hyphae do not encounter a plant root in close proximity to the spore, the mycorrhiza
will die (other types of mycorrhizae can be cultured in agar, but VA mycorrhizae do not survive without a host) (Berch and Fortin 1983). Hyphae that encounter a host root form a
structure called an appressorium, and penetrate the cell wall of the root either by mechanical pressure or through the release of cell wall-degrading enzymes (Varma 1998). Hyphae
that enter host roots through these infection points can form extensive networks both inside the root and throughout the soil surrounding the root. Some hyphae exit the root to form
runner hyphae, which track roots through the soil and can infect additional roots. Thinner, branched networks of hyphae called absorbing hyphae develop from these runner hyphae,
extending into the surrounding soil and transporting nutrients from as much as 7 cm away to the host roots (Allen 1991, Bago et al. 1998). Although a second type of mycorrhizae,
the ectomycorrhizae, tend to form more substantial networks of anastomosing hyphae, VA mycorrhizae can also form networks. When two sets of
fan-shaped hyphae meet, they can join and connect two plants of the same, or of different, species (Friese and Allen 1991). Nuclei are sometimes exchanged between fungi through
these networks, and can fuse as a form of sexual reproduction for the fungus (Allen 1991).
Intraradical hyphae, hyphae that grow within plant roots, penetrate cell walls but do not often penetrate the plasma membrane of root cells. This avoids
damage to the plant, although recently infected plants still often show an immune response to mycorrhizal infection. This system also allows for the
development of the structures for which VA mycorrhizae are named, vesicles and arbuscules. Arbuscules are short-lived structures thought to be involved in
nutrient transfer. An intraradical hyphal branch penetrates the plant cell wall, but not the plasma membrane, to form the main trunk of an arbuscule. This trunk
branches repeatedly, and the plasma membrane surrounds these branches.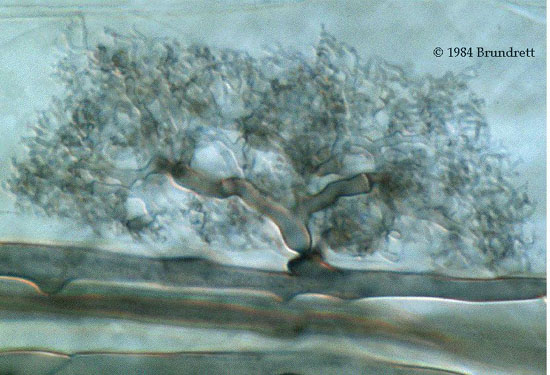 Because this area of the plasma membrane contains fewer or less highly
polymerized structural proteins, it is renamed the periarbuscular membrane (Smith and Read 1997). The periarbuscular membrane and the wall of the
arbuscule are in close contact, and plant cells containing arbuscules show an increase in metabolic activity and cytoplasm volume (Smith and Read 1997). It is not clear what nutrients
are transported through this fungal/plant interface, but phosphorus, zinc, and other soil-derived nutrients seem likely candidates (but see also Cox and Tinker 1976 in Smith and Read
1997). Arbuscules take several days to form, and in agricultural crops, often complete their entire life cycle in a week (Bevege and Bowen 1975 and Brundrett et al. 1985 in Smith
and Read 1997). Woodland and other slower-growing plants often have longer arbuscule life cycles. It is not clear why arbuscules degrade, or why their lifespan varies.
Because this area of the plasma membrane contains fewer or less highly
polymerized structural proteins, it is renamed the periarbuscular membrane (Smith and Read 1997). The periarbuscular membrane and the wall of the
arbuscule are in close contact, and plant cells containing arbuscules show an increase in metabolic activity and cytoplasm volume (Smith and Read 1997). It is not clear what nutrients
are transported through this fungal/plant interface, but phosphorus, zinc, and other soil-derived nutrients seem likely candidates (but see also Cox and Tinker 1976 in Smith and Read
1997). Arbuscules take several days to form, and in agricultural crops, often complete their entire life cycle in a week (Bevege and Bowen 1975 and Brundrett et al. 1985 in Smith
and Read 1997). Woodland and other slower-growing plants often have longer arbuscule life cycles. It is not clear why arbuscules degrade, or why their lifespan varies.
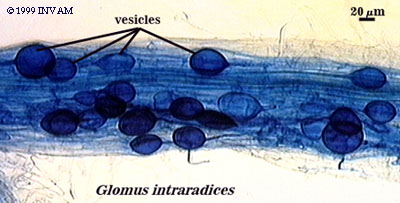
After the first arbuscules degrade, structures called vesicles often form within the infected root. Vesicles, the second characteristic structure of VA mycorrhizae, are thick-walled
structures which range in shape from oval to box-like. Vesicles contain large amounts of lipids, and often numerous nuclei. In dead root fragments, vesicles can be infective
propagules, and re-grow hyphae to infect new roots (Smith and Read 1997). As with arbuscules, little is known about the lifespan or even purpose of vesicles. Vesicles clearly play
some role in storage, and most likely are responsible for carbohydrate uptake from the host roots.
 VA mycorrhizal infection develops from either spores, root fragments, or hyphal networks in close proximity to host plant roots.
Fungal spores, in general, are spherical reproductive structures which remain dormant during periods of unfavorable conditions. Spores of VA mycorrhizae contain many nuclei (250 µm diameter spores can contain as many as 2000 nuclei (Bécard and
Pfeffer 1993 in Smith and Read 1997), and large amounts of stored lipids and carbohydrates. The purpose of this storage capacity is
still unknown, since spores do not form extensive networks of hyphae unless the hyphae contact and infect a host root. Spores
germinate in soil or in agar, and form a first hyphal tube called a germ tube. Some spores can produce multiple germ tubes at once, or produce successive germ tubes when the original is removed (Harley 1983). If it encounters a host root, and recognizes the root as such (through mechanisms not yet fully understood), the germ tube can form an appressorium and enter the root.
VA mycorrhizal infection develops from either spores, root fragments, or hyphal networks in close proximity to host plant roots.
Fungal spores, in general, are spherical reproductive structures which remain dormant during periods of unfavorable conditions. Spores of VA mycorrhizae contain many nuclei (250 µm diameter spores can contain as many as 2000 nuclei (Bécard and
Pfeffer 1993 in Smith and Read 1997), and large amounts of stored lipids and carbohydrates. The purpose of this storage capacity is
still unknown, since spores do not form extensive networks of hyphae unless the hyphae contact and infect a host root. Spores
germinate in soil or in agar, and form a first hyphal tube called a germ tube. Some spores can produce multiple germ tubes at once, or produce successive germ tubes when the original is removed (Harley 1983). If it encounters a host root, and recognizes the root as such (through mechanisms not yet fully understood), the germ tube can form an appressorium and enter the root.
Spores are just one of the possible infective VA mycorrhizal propagules. Infected root segments that have been dried and stored for as long as 6 months can infect new roots as well (Hayman 1983, Smith and Read 1997). When these root segments are placed in moist soil in close proximity to growing plant roots, new hyphae emerge from the inside of the old hyphal tubes in root segments. In some cases, old root segments may result in faster infection of plants than the addition of mycorrhizal spores, since root segments appear to produce new hyphae more quickly than spores can germinate (Hayman 1983). As described above, a third source of mycorhrizal infection is runner hyphae from plants with active mycorrhizal infection.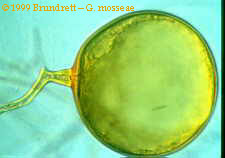
There are still a number of mysteries concerning the physiology of mycorrhizal infection, but the ecology of mycorrhizal infection is even less well understood. Hyphae form a continuous path for transfer of nutrients, carbon, and water to the host plant. Since they are considerably thinner than most plant roots, hyphae of many species of VA
mycorrhizae form extensive branching networks that reach areas of soil otherwise unavailable to their plant hosts. In effect, mycorrhizae increase the surface area of roots of the host
plants. In return, VA mycorrhizae take between 10 and 30% of the net primary production of host plants (St. John and Coleman 1983 in Allen 1991). It is estimated that mycorrhizae
cost 10% more to maintain than the equivalent area of roots, but in terrestrial systems, VA mycorrhizal infection tends to increase nutrient uptake and growth of infected plants
(Allen 1991). The benefits of VA mycorrhizae are substantial, and in addition to increased water and nutrient uptake include disease resistance, drought resistance, and greater
binding of soil particles into large aggregates and to roots (Nadian et al. 1996 in Smith and Read 1997, Sylvia in Norris et al. 1994). These benefits help explain why so many
terrestrial plants are infected by VA mycorrhizae. The remainder of this site deals with VA mycorrhizal infection in aquatic plants, an area where little is known about the ecology and
even the physiology of mycorrhizal infection, but for a fascinating book detailing the ecology of mycorrhizae in terrestrial plants, look at Allen's The Ecology of Mycorrhizae (1991).
 Because this area of the plasma membrane contains fewer or less highly
polymerized structural proteins, it is renamed the periarbuscular membrane (Smith and Read 1997). The periarbuscular membrane and the wall of the
arbuscule are in close contact, and plant cells containing arbuscules show an increase in metabolic activity and cytoplasm volume (Smith and Read 1997). It is not clear what nutrients
are transported through this fungal/plant interface, but phosphorus, zinc, and other soil-derived nutrients seem likely candidates (but see also Cox and Tinker 1976 in Smith and Read
1997). Arbuscules take several days to form, and in agricultural crops, often complete their entire life cycle in a week (Bevege and Bowen 1975 and Brundrett et al. 1985 in Smith
and Read 1997). Woodland and other slower-growing plants often have longer arbuscule life cycles. It is not clear why arbuscules degrade, or why their lifespan varies.
Because this area of the plasma membrane contains fewer or less highly
polymerized structural proteins, it is renamed the periarbuscular membrane (Smith and Read 1997). The periarbuscular membrane and the wall of the
arbuscule are in close contact, and plant cells containing arbuscules show an increase in metabolic activity and cytoplasm volume (Smith and Read 1997). It is not clear what nutrients
are transported through this fungal/plant interface, but phosphorus, zinc, and other soil-derived nutrients seem likely candidates (but see also Cox and Tinker 1976 in Smith and Read
1997). Arbuscules take several days to form, and in agricultural crops, often complete their entire life cycle in a week (Bevege and Bowen 1975 and Brundrett et al. 1985 in Smith
and Read 1997). Woodland and other slower-growing plants often have longer arbuscule life cycles. It is not clear why arbuscules degrade, or why their lifespan varies.  VA mycorrhizal infection develops from either spores, root fragments, or hyphal networks in close proximity to host plant roots.
Fungal spores, in general, are spherical reproductive structures which remain dormant during periods of unfavorable conditions. Spores of VA mycorrhizae contain many nuclei (250 µm diameter spores can contain as many as 2000 nuclei (Bécard and
Pfeffer 1993 in Smith and Read 1997), and large amounts of stored lipids and carbohydrates. The purpose of this storage capacity is
still unknown, since spores do not form extensive networks of hyphae unless the hyphae contact and infect a host root. Spores
germinate in soil or in agar, and form a first hyphal tube called a germ tube. Some spores can produce multiple germ tubes at once, or produce successive germ tubes when the original is removed (Harley 1983). If it encounters a host root, and recognizes the root as such (through mechanisms not yet fully understood), the germ tube can form an appressorium and enter the root.
VA mycorrhizal infection develops from either spores, root fragments, or hyphal networks in close proximity to host plant roots.
Fungal spores, in general, are spherical reproductive structures which remain dormant during periods of unfavorable conditions. Spores of VA mycorrhizae contain many nuclei (250 µm diameter spores can contain as many as 2000 nuclei (Bécard and
Pfeffer 1993 in Smith and Read 1997), and large amounts of stored lipids and carbohydrates. The purpose of this storage capacity is
still unknown, since spores do not form extensive networks of hyphae unless the hyphae contact and infect a host root. Spores
germinate in soil or in agar, and form a first hyphal tube called a germ tube. Some spores can produce multiple germ tubes at once, or produce successive germ tubes when the original is removed (Harley 1983). If it encounters a host root, and recognizes the root as such (through mechanisms not yet fully understood), the germ tube can form an appressorium and enter the root.
