Home
What are DNA Vaccines?
 DNA Vaccines
DNA Vaccines
Genetic/ DNA immunization is a novel technique used to
efficiently stimulate humoral and cellular immune responses to protein
antigens. The direct injection of genetic material into a living host causes
a small amount of its cells to produce the introduced gene products. This
inappropriate gene expression within the host has important immunological
consequences, resulting in the specific immune activation of the host against
the gene delivered antigen (Koprowski et al, 1998).
Traditional Vaccines: The development of
vaccination against harmful pathogenic microorganisms represents an important
advancement in the history of modern medicine. In the past, traditional
vaccination has relied on two specific types of microbiological preparations
to produce material for immunization and generation of a protective immune
response. These two categories involve either living infectious material
that has been manufactured in a weaker state and therefore inhibits the
vaccine from causing disease, or inert, inactivated, or subunit preparations.
Immunization
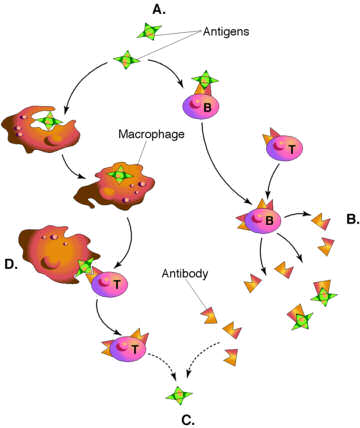 Live
attenuated vaccines stimulate protective immune responses when they replicate
in the host. The viral proteins produced within the host are released into
the extracellular space surrounding the infected cells and are then acquired,
internalized and digested by scavenger cells that circulate the body. These
cells are called antigen presenting cells (APCs)
and include macrophages, dendritic cells, and B cells, which work together
to expand immune response. The APCs recirculate fragments of the digested
the antigen to their surface, attached to
MHC
class
II antigens. This complex of foreign peptide antigen plus host MHC
class II antigens forms part of
Live
attenuated vaccines stimulate protective immune responses when they replicate
in the host. The viral proteins produced within the host are released into
the extracellular space surrounding the infected cells and are then acquired,
internalized and digested by scavenger cells that circulate the body. These
cells are called antigen presenting cells (APCs)
and include macrophages, dendritic cells, and B cells, which work together
to expand immune response. The APCs recirculate fragments of the digested
the antigen to their surface, attached to
MHC
class
II antigens. This complex of foreign peptide antigen plus host MHC
class II antigens forms part of
the specific signal  with
which APCs along with the MHC peptide complex, trigger the action of of
immune cells, the T helper lymphocytes. The second part of the activation
signal comes from the APCs themselves, which display on their cell surface
constimulatory
molecules along with MHC-antigen complexes. Both drive T call expansion
and activation through interaction with their respective ligands, the T
cell receptor complex (TCR) and the constimulatory receptors CD28/CTLA4,
present on the the T cell surface. Activated T cells secrete molecules
that act as powerful activates of immune cells. Also as viral proteins
are produced within the host cells, small parts of these proteins surface,
chaperoned by host cell MHC class I antigens.
These complexes together are recognized by a second class of T cells, killer
or cytotoxic cells. This recognition, along with other stimulation by APCs
and production of cytokine by stimulated T
cells, is responsible for the developments of mature cytotoxic
T cells (CTL) capable of destroying infected cells. In most instances
live infection induces life long immunity. Although live attenuated preparations
are the vaccines of choice they do pose the risk of reversion to their
pathogenic form, causing infection.
with
which APCs along with the MHC peptide complex, trigger the action of of
immune cells, the T helper lymphocytes. The second part of the activation
signal comes from the APCs themselves, which display on their cell surface
constimulatory
molecules along with MHC-antigen complexes. Both drive T call expansion
and activation through interaction with their respective ligands, the T
cell receptor complex (TCR) and the constimulatory receptors CD28/CTLA4,
present on the the T cell surface. Activated T cells secrete molecules
that act as powerful activates of immune cells. Also as viral proteins
are produced within the host cells, small parts of these proteins surface,
chaperoned by host cell MHC class I antigens.
These complexes together are recognized by a second class of T cells, killer
or cytotoxic cells. This recognition, along with other stimulation by APCs
and production of cytokine by stimulated T
cells, is responsible for the developments of mature cytotoxic
T cells (CTL) capable of destroying infected cells. In most instances
live infection induces life long immunity. Although live attenuated preparations
are the vaccines of choice they do pose the risk of reversion to their
pathogenic form, causing infection.
Immune
Response
In contrast, when inoculated nonlive vaccines composed
of whole or even partial viruses are not produced within the host cells,
they mainly end up in the extracellular space. They provide protection
by directly generating T helper and humoral immune
responses against the pathogenic immunogen. In the absence of the cellular
production of the foreign antigen, these vaccines are usually devoid of
the ability to induce significant T cytotoxic responses. In addition, these
vaccines are not actually produced in the host, and therefore, they are
not customized by the host. The immunity induced by their vaccines frequently
decreases during the life of the host and may require additional boosters
to achieve lifelong immunity. However, nonlive vaccines offer some important
advantages over live vaccines: they are produced earlier, and they can
be designed to contain only the specific antigenic target of the pathogen
that is involved in the development of protective immunity and exclude
all other viral components.
 Genetic Immunization: Since its early applications
in the 1950's, DNA-based immunization has become a novel approach to vaccine
Genetic Immunization: Since its early applications
in the 1950's, DNA-based immunization has become a novel approach to vaccine
development. Direct injection of naked plasmid DNA induces
strong immune responses to the antigen encoded by the gene vaccine. Once
the plasmid DNA construct is injected the host cells take up the foreign
DNA, expressing the viral gene and producng the corresponding viral protein
inside the cell. This form of antigen presentation and processing induced
both MHC and class I and class II restricted cellular and humoral immune
responses (Encke, J. et al, 1999).
-
History: The use of genetic material to deliver
genes for therapeutic purposes has been practiced for many years. Experiments
outlining the transfer of DNA into cells of living animals were reported
as early as 1950. Later experiments using purified genetic material only
further confirmed that the direct DNA gene injection in the absence of
viral vectors results in the expression of the inoculated genes in the
host. There have been additional experiments that extend these findings
to recombinant DNA molecules, further illustrating the idea that purified
nucleic acids could be directly delivered into a host and proteins would
be produced. In 1992, scientists Tang and Johnson reported that the delivery
of human growth hormone in a expression cassette in vivo resulted
in production of detectable levels of the growth hormone in host mice.
They also found that these inoculated mice developed antibodies against
the human growth hormone; they termed this immunization procedure "genetic
immunization", which describes the ability of inoculated genes to be individual
immunogens (Koprowski et al, 1998).
DNA Vaccines
-
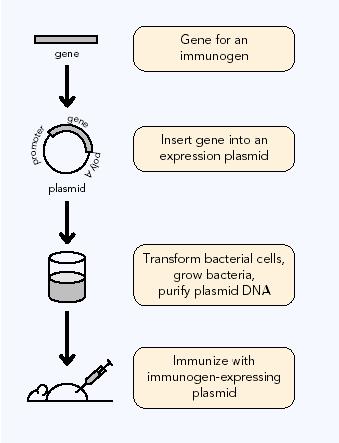
Construction: DNA vaccines are composed
of a bacterial plasmids. Expression plasmids used in DNA-based vaccination
normally contain two unites: the antigen expression unit composed of promoter/enhancer
sequences, followed by antigen-encoding and polyadenylation sequences and
the production unit composed of of bacterial sequences necessary for plamid
amplification and selection (Schirmbeck, R., 2001). The construction of
bacterial plasmids with vaccine inserts is accomplished using recombinant
DNA technology. Once constructed, the vaccine plasmid is transformed
into bacteria, where bacterial growth produces multiple plasmid copies.
The plasmid DNA is then purified from the bacteria, by separating the circular
plasmid from the much larger bacterial DNA and other bacterial impurities.
This purifies DNA acts as the vaccine (AAM,
1996).
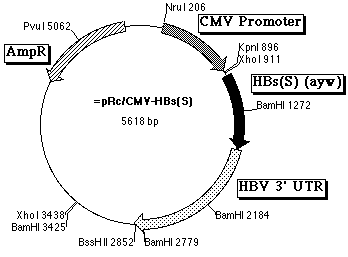
DNA vaccine plasmid
-
Administration- Over the past decade of clinical
research and trials, several possible routs of plasmid delivery have been
found. Successful immunization has been demonstrated after delivery of
plasmids through intramuscular, intradermal and intravenous injection.
The skin and mucous membranes being considered the best site for immunization
due to the high concentrations of dendritic cells (DC), macrophages and
lymphocytes (Raz,E., 1998). Intradermal injection
of DNA-coated gold particles with a gene gun have been used. The plasmid
DNA can be diluted in distilled water, saline or sucrose. There has also
been positive demonstration of proinjection or codelivery with various
drugs (Encke et al, 1999).
-
Mechanisms: A plasmid vector that expresses
the protein of interest (e.g. viral protein) under the control of an appropriate
promoter is injected into the skin or muscle of the the host. After uptake
of the plasmid, the protein is produced endogenously and intracellularly
processed into small antigenic peptides by the host proteases. The peptides
then enter the lumen of the endoplasmic reticulum (E.R.) by membrane-associated
transporters. In the E.R., peptides bind to MHC class I molecules.
These peptides are presented on the cell surface in the context of the
MHC class I. Subsequent CD8+ cytotoxic T
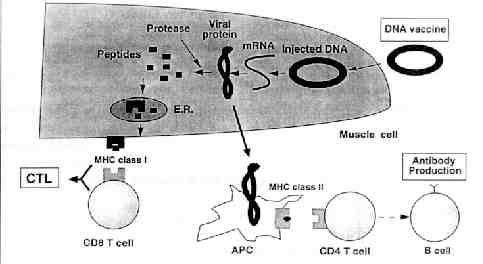 cells
(CTL) are stimulated and they evoke cell-mediated immunity. CTLs inhibit
viruses through both cytolysis of infected cells and noncytolysis mechanisms
such as cytokine production (Encke et al, 1999).
cells
(CTL) are stimulated and they evoke cell-mediated immunity. CTLs inhibit
viruses through both cytolysis of infected cells and noncytolysis mechanisms
such as cytokine production (Encke et al, 1999).
The foreign protein can also be presented
by the MHC class II pathway by APCs which elicit helper T cells (CD4+)
responses. These CD4+ cells are able to recognize the peptides formed from
exogenous proteins that were endocytosed or phagocytosed by APC, then degraded
to peptide fragments and loaded onto MHC class II molecules. Depending
on the the type of CD4+ cell that binds to the complex, B cells are stimulated
and antibody production is stimulated. This is the same manner in which
traditional vaccines work (Schirmbeck et al.,
2001).
DNA Vaccine Mechanism
-
Advantages: DNA immunization offers many advantages
over the traditional forms of vaccination. It is able to induce the expression
of antigens that resemble native viral epitopes more closely than standard
vaccines do since live attenuated and killed vaccines are often altered
in their protein structure and antigenicity. Plasmid vectors can be constructed
and produced quickly and the coding sequence can be manipulated in
many ways. DNA vaccines encoding several antigens or proteins can be delivered
to the host in a single dose, only requiring a microgram of plasmids to
induce immune responses. Rapid and large-scale production are available
at costs considerably lower than traditional vaccines, and they are also
very temperature stable making storage and transport much easier. Another
important advantage of genetic vaccines is their therapeutic potential
for ongoing chronic viral infections. DNA vaccination may provide
an important tool for stimulating an immune response in HBV, HCV and HIV
patients. The continuos expression of the viral antigen caused by gene
vaccination in an environment containing many APCs may promote successful
therapeutic immune response which cannot be obtained by other traditional
vaccines (Encke et al, 1999). This is a subject
that has generated a lot of interest in the last five years.
-
Limitations: Although DNA can be used to raise
immune responses against pathogenic proteins, certain microbes have outer
capsids that are made up of polysaccharides. This limits the extent
of the usage of DNA vaccines because they cannot substitute for polysaccharide-based
subunit vaccines (AMM, 1996).
-
Future- It has recently been discovered that
the transfection of myocytes can be amplified by pretreatment with local
anesthetics or with cardiotoxin, which induce local tissue damage and initiate
myoblast regeneration. Gaining a full understanding of this mechanism of
DNA uptake could prove helpful in improving applications for gene therapy
and gene vaccination. Both improved expression and better engineering of
the DNA plasmid may enhance antibody response to the gene products and
expand the applications of the gene vaccines (Raz, E.,
1998).
Back
Next


 Live
attenuated vaccines stimulate protective immune responses when they replicate
in the host. The viral proteins produced within the host are released into
the extracellular space surrounding the infected cells and are then acquired,
internalized and digested by scavenger cells that circulate the body. These
cells are called antigen presenting cells (APCs)
and include macrophages, dendritic cells, and B cells, which work together
to expand immune response. The APCs recirculate fragments of the digested
the antigen to their surface, attached to
MHC
class
II antigens. This complex of foreign peptide antigen plus host MHC
class II antigens forms part of
Live
attenuated vaccines stimulate protective immune responses when they replicate
in the host. The viral proteins produced within the host are released into
the extracellular space surrounding the infected cells and are then acquired,
internalized and digested by scavenger cells that circulate the body. These
cells are called antigen presenting cells (APCs)
and include macrophages, dendritic cells, and B cells, which work together
to expand immune response. The APCs recirculate fragments of the digested
the antigen to their surface, attached to
MHC
class
II antigens. This complex of foreign peptide antigen plus host MHC
class II antigens forms part of
 with
which APCs along with the MHC peptide complex, trigger the action of of
immune cells, the T helper lymphocytes. The second part of the activation
signal comes from the APCs themselves, which display on their cell surface
constimulatory
molecules along with MHC-antigen complexes. Both drive T call expansion
and activation through interaction with their respective ligands, the T
cell receptor complex (TCR) and the constimulatory receptors CD28/CTLA4,
present on the the T cell surface. Activated T cells secrete molecules
that act as powerful activates of immune cells. Also as viral proteins
are produced within the host cells, small parts of these proteins surface,
chaperoned by host cell MHC class I antigens.
These complexes together are recognized by a second class of T cells, killer
or cytotoxic cells. This recognition, along with other stimulation by APCs
and production of cytokine by stimulated T
cells, is responsible for the developments of mature cytotoxic
T cells (CTL) capable of destroying infected cells. In most instances
live infection induces life long immunity. Although live attenuated preparations
are the vaccines of choice they do pose the risk of reversion to their
pathogenic form, causing infection.
with
which APCs along with the MHC peptide complex, trigger the action of of
immune cells, the T helper lymphocytes. The second part of the activation
signal comes from the APCs themselves, which display on their cell surface
constimulatory
molecules along with MHC-antigen complexes. Both drive T call expansion
and activation through interaction with their respective ligands, the T
cell receptor complex (TCR) and the constimulatory receptors CD28/CTLA4,
present on the the T cell surface. Activated T cells secrete molecules
that act as powerful activates of immune cells. Also as viral proteins
are produced within the host cells, small parts of these proteins surface,
chaperoned by host cell MHC class I antigens.
These complexes together are recognized by a second class of T cells, killer
or cytotoxic cells. This recognition, along with other stimulation by APCs
and production of cytokine by stimulated T
cells, is responsible for the developments of mature cytotoxic
T cells (CTL) capable of destroying infected cells. In most instances
live infection induces life long immunity. Although live attenuated preparations
are the vaccines of choice they do pose the risk of reversion to their
pathogenic form, causing infection.
