
Alzheimer's disease (AD) is a progressive degenerative disease of the brain marked by gradual and irreversible declines in cognitive functions. Cognitive impairments associated with Alzheimer's suggest a dysfunction in the cholinergic system. This hypothesis is strengthened by postmortem data collection, indicating low cholinergic activity.
The cholinergic hypothesis led to the prediction that cholinesterase inhibitors would reverse the deficit in acetylcholine levels associated with AD, and consequently reverse the memory impairments characteristic of the disease.
Acetylcholinesterase (AChE) is the cholinergic enzyme that is responsible for the hydrolysis of acetylcholine. It is found at the synapse between nerve cells and muscle cells.
Aricept (E2020) < > was approved by the FDA in 1996 for the treatment of Alzheimer's disease. It acts by reversibly inhibiting acetylcholinesterase from hydrolyzing acetylcholine, which, in turn, increases the availability of this neurotransmitter to the brain and strengthens nerve signals [2].
The hydrolytic function of acetylcholinesterase occurs at the bottom of a 20 angstrom deep active site gorge < > ( includes a top view of Aricept entering the active site gorge). The active site gorge of acetylcholinesterase consists of five distinct subunits: the peripheral binding site < > , the anionic binding site < > , the acyl pocket < >, the oxyanion hole < >, and the catalytic triad < >. The entire gorge is composed of fourteen conserved amino acid residues, more than half of which contain aromatic rings [3]. The three major functional groups of Aricept interact with the aromatic rings of the gorge via water mediated pi-pi interactions. The three major functional groups are the benzyl moiety, the piperidine nitrogen, and the dimethoxyindanone moiety [2] .

The benzyl ring interacts primarily with Trp 84 ; the piperidine ring interacts with Tyr 70, Tyr 121, and Tyr 334 ; and the indanone ring interacts with Trp 279 at the entrance of the gorge. These contacts are essential for the favorable and selective binding of E2020 with acetylcholinesterase [4].
Aricept orients itself along AChE by extending from the anionic site, at the bottom of the active site, to the peripheral binding site, at the top entrance to the active-site gorge.
Aricept's high affinity and selectivity for AChE is primarily due to the structure of the peripheral binding site and the interactions that occur there. Specifically, Aricept's indanone ring stacks against the ring of Trp 279 via hydrogen bonding and Van der Waals contacts. The hydrogen bonds throughout the entire active site, however, are essential in giving Aricept such high affinity for AChE [2].
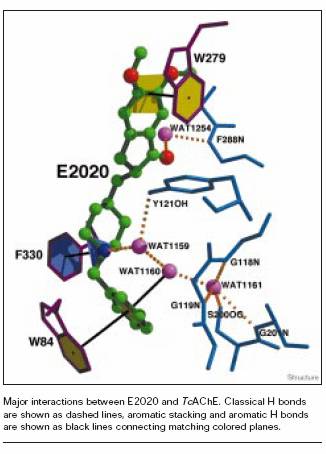
Affinity is also enhanced through Aricept's interaction with the anionic binding site, namely the aromatic residue involved in recognition of acetylcholine, Trp 84. Furthermore, an aromatic residue situated at the midpoint of the gorge, Phe330 , also contributes to Aricept's affinity for AChE.
Twenty-five water molecules can be seen within the gorge of the Aricept-AChE complex. These water molecules are largely conserved in analogs of the Aricept-AChE complex. When comparing the Aricept-AChE complex to its analogous structures, it is evident that three water molecules have been introduced to the structure, thereby suggesting that these solvent molecules are of importance.
Aricept displaces these three molecules, as well as five other water molecules that are present in the native AChE structure, in order to fit into the gorge [2].

Although five conserved molecules are displaced, it has been shown that the majority of the conserved waters simply adhere to the gorge wall.
Aricept's structure is relatively rigid in its interactions with the gorge of AChE. Therefore, it is believed that sites with conformational flexibility play critical roles in Aricept's function. It has been shown that Aricept has two rotatable bonds on each side of its piperidine functional group, which are partially responsible in conferring Aricept with high affinity for AChE. Aricept must also be flexible with respect to Phe330, which acts as a swinging gate, swinging out of the way towards the gorge wall. It is thought that the swinging gate may serve to guide acetylcholine towards the active site, while simultaneously isolating the reaction center from the rest of the gorge [2].
The oxyanion hole < >, the catalytic triad < > and the acyl pocket < > do not interact directly with AChE, but only indirectly, via water molecules < >. The catalytic triad is the reaction center of the enzyme, where the catalysis of acetylcholine occurs. The oxyanion hole and the acyl pocket provide spaces into which substituents could fit, thereby creating the possibility that new and more effective drugs may be generated through future modification of Aricept at these sites [2].
In addition to AChE, Aricept also binds to butyrylocholinesterase (BChE), another cholinergic enzyme. It is thought, however, that inhibition of BChE causes adverse side effects. Other drugs used in the treatment of AD not only show equal affinities for AChE and BChE but also must be administered up to four times a day, thereby creating an increased risk of hepatotoxicity (state of toxic damage to the liver) [1]. Aricept shows a selectivity for AChE that is 1000 times greater than for BChE, thus making Aricept a preferred drug choice [2].
Aricept is able to demonstrate such high affinity and specificity for AChE as a result of its interactions at the active site of the enzyme. As explained earlier, three major interaction sites are largely responsible for giving Aricept this enhanced ability: the anionic site (Trp 84), the gorge midpoint (Phe330), and the peripheral site (Trp279) < > [3]. It has been found that Phe330 and Trp 279 are conserved in AChE, but absent in BChE, suggesting that Aricept’s selectivity for AChE as opposed to BChE arises from the structural differences at these enzyme sites, particularly that at the peripheral site. Therefore, recent evidence suggests that differential specificity is due to structural differences in the active sites of AChE and BChE, and not to spatial differences, as was previously believed [2].
[1] Greenblatt, Harry M., et al. Acetylcholinesterase: A Multifaceted Target for Structure-Based Drug Design of Anticholinesterase Agents for the Treatment of Alzheimer's Disease. The Journal of Molecular Neuroscience, Vol. 20, No. 3, August 2003.
[2] Kryger, Gitay, et al. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure, Vol. 7, No. 3, March 1999.
[3] Kryger, Gitay, et al. Three-dimensional structure of a complex of E2020 with acetylcholinesterase from Torpedo californica. Journal of Physiology-Paris, Vol. 92, No. 3-4, June 1998.
[4] Saxena, Ashima, et al. Aromatic amino-acid residues at the active and peripheral anionic sites control the binding of E2020 (Aricept) to cholinesterases. European Journal of Biochemistry, Vol. 270, No. 22, Nov. 2003.