Insulin Interactions
with the Insulin Receptor
Taylor Maurer '17 and Morgan Perrett '17
Contents:
I. Introduction
Insulin, an important hormone in the endocrine
system, regulates and maintains carbohydrate metabolism, promoting
cell growth and function. When Insulin interacts with the
,
a part of the Insulin Receptor (IR), it triggers a phosphorylation
cascade starting in the holoreceptor's tyrosine kinase
domain. This leads to the introduction of glucose into the
cell. Without Insulin, glucose is prevented from entering the
cell; thus, Insulin's interaction with the IR regulates the
intracellular concentration of glucose. Until recently, the
Insulin-IR binding mechanism was unknown. We will uncover the
newly discovered mechanisms between Insulin and its receptor by
highlighting the interactions that solidify its binding.
II. General Structure
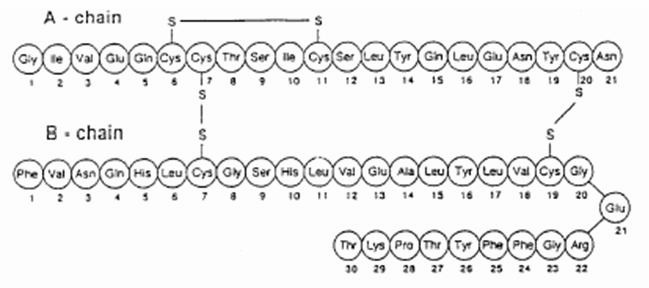
Insulin is stored as a zinc-coordinated
hexamer. However, this hexamer dissociates into zinc-free
monomers that are able to bind to the IR. A single Insulin
monomer has two chains -
and
- that are connected by three
disulfide bonds (Figure 1), one of which is an intramolecular
disulfide bond on Chain A.
Both of these chains are needed for Insulin to interact with its
receptor.
Figure 1: Disulfide Bonds in an Insulin Monomer
The Insulin receptor is a heterotetramer consisting of multiple
subunits. Each IR monomer includes an alpha-subunit leucine-rich
repeat domain (L1 Beta Two Sheet) combined with a
cysteine-rich domain (CR)
,
as well as an alpha-subunit C-terminal segment (alpha-CT)
.
These are located in the extracellular matrix and constitute the
.
The
supplementary image shows an additional leucine-rich repeat
domain (L2) and the first, second, and third fibronectin type III
domains, which, combined with the microreceptor, constitute the
holoreceptor. There are two isoforms of the Insulin receptor:
IR-A and IR-B. IR-A has an additional 12 amino acids on the
C-terminal of the alpha-CT
subunit. The displayed protein is in the IR-A form.
The two extracellular subunits -L1 Beta
Two Sheet and alpha-CT-
bind a single Insulin monomer. This results in a change in
conformation in both the Insulin monomer and the IR's holoreceptor,
which initiates the aforementioned phosphorylation cascade.
III. Insulin Conformation and Stability
Before Insulin can bind to the microreceptor, it must change
conformation. Insulin has two conformations: an active
conformation used in binding and a free conformation. If Insulin
does not change conformation, there is a steric clash between the alpha-CT subunit and the B25-B30
residues. The change between the two forms is mediated by two
"hinge-like" rotations at the
. Specifically, the hormone rotates approximately 10 degrees
around the
residue, followed by a 50 degree turn -known as the B26 turn because
of B26's crucial role- around the
residue. After
both of the aforementioned rotations,
is anti-parallel to the first strand of
the L1
Beta Two Sheet and perpendicular to the Chain B
alpha-helix .
Simultaneously, the alpha-CT helix
extends to include residues 711-714,
the alpha-CT helix between the L1 Beta Two Sheet and Insulin's Chain A. Therefore, after the two
"hinge-like" rotations, the 705-714 residues in the alpha-CT
helix occupy the space previously occupied by B25-B30 residues in the
free hormone.
The B26 turn is stabilized and maintained by
involving TyrB26. One
hydrogen bond involves a water-mediated reaction between TyrB26
and the backbone of GlyB8, while
in the other TyrB26 interacts
with the backbone of PheB24.
The importance of these hydrogen bonds, and thus TyrB26's
presence, to the 50 degree turn was examined by Zakova et al.
(2014). In order to demonstrate the importance of the TyrB26
side chain hydroxyl, they substituted Phenylalanine into position
B26. This mutation resulted in a 50% decrease in Insulin's
binding affinity and highlights the importance of TyrB26's
two hydrogen bonds to the backbone of GlyB8
and PheB24.
These hydrogen bonds are important for stabilizing and maintaining the
rotations necessary for Insulin to assume the active conformation.
Furthermore, in order for the active form of Insulin to bind to its
receptor, the Insulin monomer must be stable. In particular, the
stability of the N-terminal A chain
alpha helix
is crucial for the correct placement of many hormone receptor
contacts. Any mutations that cause a distortion of this helix
will inhibit correct binding. This helix is
by the packing of ValA3,
IleA2, as well as Chain A's intramolecular disulfide bond.
The importance of
to the stability of the N-terminal A
chain alpha helix was determined using several amino acid
substitutions. Xu et al. (2002) found that when IleA2
is substituted with Alanine, the N-terminal
A chain alpha helix undergoes segmental unfolding, which
inhibits correct binding. Furthermore, the importance of ValA3 was already well known due to
naturally occurring mutations. In response, Huang et al. (2007)
studied ValA3's
contribution to the stability of the Insulin molecule. They
first converted ValA3 to a
smaller entity: alpha-aminobutyric acid (Aba, Figure 2).
Figure 2: Alpha-aminobutyric Acid (Aba) Bond-line Structure

Despite fitting nicely into the Chain A-Chain B
that ValA3
typically resides in, AbaA3 Insulin had decreased stability.
Aba's lack of ValA3's gamma methyl group
created fewer opportunities for hydrophobic interactions in the mostly
nonpolar crevice. Next, Huang et al. determined how a polar
moiety would affect stability by creating ThrA3 Insulin. ThrA3
Insulin's N-terminal A chain alpha
helix was also less stable than ValA3.
This shows the significance of nonpolar packing in the Chain
A-Chain
B crevice ValA3 resides
in.
IV. Insulin Binding
How Insulin interacts with the IR is still an area of
investigation. Researchers currently use multiple techniques,
including mutagenesis and photo-crosslinking to crystallized
mini-receptors, to study this complicated molecule. Due to the
complexity of Insulin-IR binding, every crucial interaction used in
the binding of Insulin to the IR could not be expanded upon
here. However, the following section highlights some of the
interactions pivotal to the binding of Insulin to its receptor.
Many of the residues that play an essential role in Insulin-IR
binding are found in Site 1, a grouping of residues defined by Zakova
et al. (2014) to be responsible for effective IR binding. After
Insulin's two rotations,
- which contains GlyA1,
IleA2, ValA3, GlnA5,
TyrA19
on Chain A, and ValB12,
LeuB11, PheB24,
and PheB25 on Chain
B- is exposed. Even though the all of the exact
interactions and conformations of these residues are unclear, it is
certain they insert themselves between the alpha-CT
subunit and the L1 Beta Two Sheet.
They can then interact with the microreceptor using
.
Specifically, many of the interactions between Insulin and the IR
occur when IR residues insert themselves in nonpolar pockets created
by Site 1 residues. For example, Phe714 (not available) in the alpha-CT subunit inserts itself into
a
formed by GlyA1,
IleA2,
TyrA19, LeuB11, and ValB12.
Hydrophobic interactions like this help hold the Insulin molecule and
IR together.
Furthermore, the aromatic nature of some residues is of great
importance. Specifically, PheB25's
side chain projects away from the L1
Beta Two Sheet, which allows for its insertion into a
shallow pocket located in the alpha-CT
between Pro718 and Val715.
The aromatic portion of
is also crucial. Kristensen et al. (1996) found that the creation of
LeuA19 Insulin reduced binding 1000 fold, while the creation of PheA19
Insulin only reduced binding affinity 4 to 5 fold. This
indicates TyrA19's
aromatic ring is crucial for Insulin-IR interactions. Another
important aromatic residue is PheB24.
Its aromatic ring projects into a hydrophobic pocket, where it can
interact using
with residue Phe714,
as well as B-chain residues ValB12,
LeuB15,
and TyrB26.
Non-aromatic residues are also crucial for Insulin binding. For example, ValA3,
which also plays a large role in stabilizing Insulin.
Photo-crosslinking studies by Huang et al. (2007) show that the
orientation of the residue in the Chain A-Chain B
crevice allows it to interact using van der waals interactions with
,
encompassed in the alpha-CT
subunit.
V. Implications
Understanding the residues essential to Insulin-IR binding sheds
light on the causes of certain diseases, such as Diabetes
Mellitus. As demonstrated above, many of Insulin's hydrophobic
and aromatic residues must be maintained to retain proper binding and
engagement with its receptor. Insulin's reduced binding affinity
is determental to the life of the cell and the organism. These
findings are an invaluble tool for the design of more effective
Insulin analogs, as well as new drug therapies.
According to the National Center of Chronic Disease Prevention and
Health Promotion, 29.1 million Americans suffer from Type 1 and Type 2
Diabetes Mellitus. Fortunately, Insulin analogs can be
administered by injection to lower elevated blood sugar levels in the
body. However, eliminating this life altering disease should
remain the focus of healthcare professionals, reseachers and
patients. The research of the included authors contributes
greatly to the understanding of this hormone and its receptor. With
further research, a cure for Diabetes is on the horizon.
VI. References
Diabetes Latest.
June 17, 2014. National Center of Chronic Disease Prevention and
Health Promotion. December 16, 2015.
<http://www.cdc.gov/features/diabetesfactsheet/>
Stevan R.
Hubbard. 1997. Crytstal structure of the activated insulin
receptor tryosine kinase in complex with peptide substrate and ATP
analog. The EMBO Journal
16: 5573-5581.
Kun Huang, Shu
Jin Chan, Qing-xin Hua, et al. 2007. The A-chain of Insulin
Contacts the Insert domain of the Insulin Receptor. The
Journal of Biological Chemistry 282.48: 35337-35349.
Lucie Kosinova,
Vaclav Veverka, Pavlina Novotna, et al. 2014. Insight into the
Structural and Biological Relevance of the T/R Transistion of the
N-Terminus of the B-Chain in Human Insulin. The
American Chemical Society 53: 3392-3402.
Claus Kristensen,
Thomas Kjeldsen, Finn c. Wiberg, et al. 1996. Alanine Scanning
Mutagenesis of Insulin. The
Journal of Biological Chemistry 272.20: 12978-12983.
John G. Menting, Jonathon Whittatker, Mai B. Margetts,
et al. 2013. How Insulin engages its primary binding site on the
Insulin receptor. Nature
493.7431: 241-245.
John G.
Menting, Yanwu Yang, Shu Jin Chan, et al. 2014. Protective hinge in
Insulin opens to enable its receptor engagement. Proceedings
of the National Academy of Sciences of the United States of America
E3595 - E3404.
Bin Xu, Qing-xin
Hua, Satoe G. Nakagwa. 2002. Chiral mutagenesis of Insulin's
hidden receptor-binding surface: structure of an Allo-Isoleucine
A2 analouge. Journal of
Molecular Biology 316.3: 435-441.
Lenka Zakova,
Emilia Klevikova, Martin Lepsik, et al. 2014.
Human insulin analogues modified at the B26 site reveal a hormone
conformation that is undetected in the receptor complex. Acta
Crystallographica D70: 1001-1007.
Back to Top