ľ-opioid Receptor
Taylor Jamil '17 and Eliana McCann Smith '17
Contents:
I. Introduction
Opioids are the most broadly used analgesic drug to treat pain
and other disorders such as diarrhea, cough, post-operative pain, and
cancer. Although there are three types of opioid receptors (Mu, Delta,
and Kappa), ľ-opioid receptors (ľOR) are the most commonly activated
receptors in pain-associated pathways. ľOR are G-protein coupled
receptors (GPCR) that contain 7-transmembrane domains. These opioid
receptors are bound to the endoplasmic reticulum. Upon activation by a
ligand, the ľOR translocates to the membrane in order to block
potassium channels which causes cellular hyperpolarization. This in
turn prevents the neuron from reaching its threshold for sending an
action potential, thus preventing pain signals from being transmitted.
The G-proteins have a GTP attached to them, which is hydrolyzed to
GDP+Pi upon ligand binding. The protein will detach from the receptor
and interact with effectors, such as cAMP downstream to
amplify the signal. The most common endogenous ligand are
beta-endorphins. Agonists include morphine and fentanyl and are
synthesizable opioid ligands that provide the same endogenous effects
as endorphins.
In addition to the analgesic properties of these drugs, many opioid ligands such as
morphine also activate the central dopamine pathways providing a
sense of euphoria. This unwanted side-effect creates a lot of
addictive behaviors, which drives researchers to look for other
alternatives without euphoric effects. Further, a large difficulty in
drug development is synthesizing a ligand that binds well to only the
desired target receptor. Using crystal structures to learn how
receptors convert from the inactive state to the active state upon
ligand binding serves to address how well a receptor interacts with
its substrate. Huang et al. (2015) manipulated ligand structures to
develop a better understanding as to how the ligand affects the
transition of ľOR from its
inactive to active state. However, we focus on the ligand, BU72
(4VO) in the activated ľOR state.
II. General Structure
There are 4 extracellular and
4 cytoplasmic hydrophilic protein
chains, and 7 helical transmembrane hydrophobic
chains.
The 7 transmembrane alpha-helices, 4 extracellular loops, and 4
cytoplasmic loops in ľOR contain the amino terminus on the
extracellular end and the carboxyl on the cytoplasmic end. The extracellular
(ECL1-4) and cytoplasmic (CL1-4)
protein regions are hydrophilic, whereas the transmembrane regions
are hydrophobic (TM1-7).
The ľ-opioid receptor is a G-protein coupled receptor, however this
complex has proven too difficult to crystallize when bound to a
ligand. Instead, researchers were able to use a G-protein mimic in
the form of a nanobody to crystallize
the protein in its active conformation.
III.G-Protein Stabilization
Since the ľOR is a GPCR, the receptor is not stable without
the G-protein. Since the G-protein could not be crystallized,
researchers crystalized the ľOR with a camelid single-domain
antibody that mimics the G-protein.
There are both hydrophobic and polar interactions between the
two proteins, where the blue represents the hydrophobic
interactions.
In the crystal structure, the nanobody also
interacts with nanobodies of additional receptor-nanobody complexes. Based on the contacts
between ľOR and the G-protein mimic, one can see that the
nanobody does not interlace deeply into the ľOR intracellular
domain. The hydrogen bonds formed are between the nanobody's
residues
I56, P58, T59, T69, S71, V102 and the ľOR residues
R179, N342, L176, P172, E270 in the CL2, Helix 8,
and CL3 domains.
IV. Agonist Ligand Binding
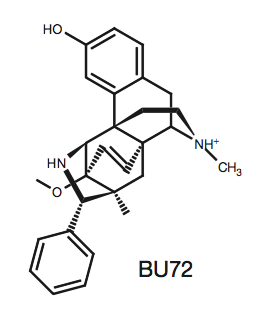 Bu72
(4VO)
is a morphine-like agonist that induces similar effects to
endogenous beta-endorphins. When BU72 binds to ľOR, the receptor
transitions into its active state. The interactions between the
ligand and receptor are vital in drug development because the
specific residues in the ligand binding pocket and their distances
apart should be taken into consideration when designing substrates
for the receptor. Most of the interactions in the ligand binding
pocket are hydrophobic between the aromatic, phenolic groups of BU72
and residues: Y148, V236, V300, I296,
W318, I322, Y326, W133, V143, and I144.
The white on BU72 indicate atoms not interacting with the receptor's hydrophobic residues because they are nitrogen or oxygen.
Instead, these atoms form the two polar interactions.
The first interaction is water mediated between Y148,
K233, H297, two
water molecules, and the phenolic hydroxyl
on BU72. The second polar bond is between Y326,
D147, and the tertiary amino group
on BU72. Additionally, the extracellular amino terminus forms a lid,
covering U72 in the ligand binding pocket. The amino terminus
residue H54 interacts with the ligand to form the lid, however,
mutation of this residue shows that it is not sufficient for lid
formation, meaning there are multiple other factors aiding to this
conformation.
Bu72
(4VO)
is a morphine-like agonist that induces similar effects to
endogenous beta-endorphins. When BU72 binds to ľOR, the receptor
transitions into its active state. The interactions between the
ligand and receptor are vital in drug development because the
specific residues in the ligand binding pocket and their distances
apart should be taken into consideration when designing substrates
for the receptor. Most of the interactions in the ligand binding
pocket are hydrophobic between the aromatic, phenolic groups of BU72
and residues: Y148, V236, V300, I296,
W318, I322, Y326, W133, V143, and I144.
The white on BU72 indicate atoms not interacting with the receptor's hydrophobic residues because they are nitrogen or oxygen.
Instead, these atoms form the two polar interactions.
The first interaction is water mediated between Y148,
K233, H297, two
water molecules, and the phenolic hydroxyl
on BU72. The second polar bond is between Y326,
D147, and the tertiary amino group
on BU72. Additionally, the extracellular amino terminus forms a lid,
covering U72 in the ligand binding pocket. The amino terminus
residue H54 interacts with the ligand to form the lid, however,
mutation of this residue shows that it is not sufficient for lid
formation, meaning there are multiple other factors aiding to this
conformation.
V. References
Chen Y, Mestek A, Liu J, Hurley JA, and
Yu L. (1993). Molecular cloning and functional expression of
a mu-opioid receptor from rat brain. Molecular
Pharmacology, 44(1): 8-12.
Huang W, Manglik A, Venkatakrishnan
AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE,
Livingston KE, Thorsen TS, Kling RC, Granier S, Gmeiner P,
Husbands SM, Traynor JR, Weis WI, Steyaert J, Dror RO, and
Kobilka BK. (2015). Structural insights into ľ-opioid
receptor activation. Nature, 524, 315-321.
Zubieta JK, Smith YR, Bueller JA, Xu
Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, a nd
Stohler CS. (2015). Regional Mu Opioid Receptor Regulation
of Sensory and Affective Dimensions of Pain. Science,
293, 331-315.
European College of
Neuropsychopharmacology. (2007, October 15). How Does the
Opioid System Control Pain, Reward and Addictive Behavior?
ScienceDaily. Retrieved December 13, 2015 from
www.sciencedaily.com/releases/2007/10/07014163647.htm
Back to Top