Human Naa60
Ellen Corcoran '18 and Julia Josowitz '18
Contents:
I. Introduction
Model View:
Color Scheme:
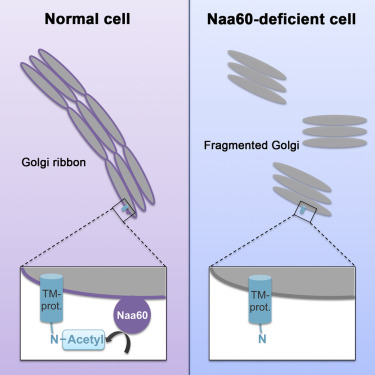
The Human Naa60 is a N-terminal acetyltransferase
.It is only found in multicellular eukaryotes and mainly catalyzes the
N-terminal acetylation of transmembrane proteins. It is located on the
Golgi apparatus through its C-terminal membrane region. It plays a
role in maintaining the integrity of the Golgi apparatus, as knockdown
results in fragmentation of the organelle. It also has lysine NE
acetyltransferase (KAT) activity, which allows it to facilitate
acetylation of free histone H4. Thus, Naa60 also plays a role in
nucleosome assembly. Knockdown of this protein inhibits cell
proliferation and induces apoptosis.
II. General Structure
Model View:
Color Scheme:
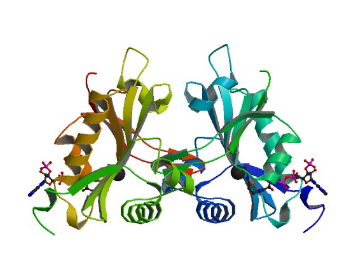
Naa60 is an enzyme containing a Gcn5-related
N-acetyltransferase (GNAT) fold. The monomer is made up of
nine beta strands and nine
alpha helixes,
resembling the structure of other N-acetyltransferases. Naa60
differs from these other NATs with a 20 residue
loop that forms a small subdomain that is critical for
protein stabilization. Sequence divergence also occurs at the
which is linked to protein dimerization.
However, the enzyme is only active in it monomeric state and
dimerization contributes to inactivation. The beta seven and beta
eight strands of Naa60 are also significantly different from the
structure of other NATs, with an antiparallel beta-hairpin
structure. The helical structures formed by the N and C-terminals
reach out from the central GCN5 domain. The
is amphiphatic, and contacts a neighbor molecule through hydrophobic
interactions between the alpha five helix
and a hydrophobic groove between the N-terminal beta-1 and
beta-3 strands of the neighboring molecule.
A malonate molecule is located in the active site and may be
indicative of the substrate binding position of Naa60. Residues Tyr
38, Asn
143, Tyr 165 interact with
the malonate through hydrogen bonds or water bridges
. The active site of Naa60 includes residues Glu
37, Tyr 97, and His
138
.
Residues 182-216 are important for
localization on the Golgi apparatus,
and specifically the alpha five helix,
made of residues 190 to 202
This helix is made up of hydrophobic residues Ile
190, Leu 191, Ile
194, Leu 197, and Leu
201
.
III. Protein Dimerization
Model View:
Color Scheme:
Here, Naa60 is a monomeric enzyme in complex with Acetyl
CoA. However, the hNaa60 specific
allows the molecule to dimerize. The loop of the first
protomer protrudes into the calaytic site of the second protomer in
the dimer when no substrate peptide is present. Subseqeuntly,
substrates are unable to bind and the enzyme becomes inactive.
Several contacts mediate hNaa60/CoA dimerization, primarily those
that anchor peptide substrates in the hNaa60 monomer. Van der Waals
contacts connect Leu 171 of each dimer.
IV. Localization on the Golgi Apparatus and Protein
Differentiation
Model View:
Color Scheme:
In HeLa cells, the knockdown of Naa60 results in Golgi
Apparatus fragmentation, which shows the importance of the protein
in the integrity of this organelle. Comparison of hNaa60 to Naa50
suggests that the sequential differences in the
are responsible for hNaa60's role in the functionality of
the Golgi Apparatus. More specifically, the amphipathic
helix
accounts for the interaction between hNaa60 and the Golgi
membrane due to its ability to interact with neighboring units via
hydrophobic interactions.
V. Activating Regions
Model View:
Color Scheme:
Recent studies suggest that
is crucial to the proper positioning of Acetyl CoA transfer
and facilitates catalytic activity. The malonate
molecule indicates a substrate binding site of hNaa60,
where Trp 33 , Tyr
38 , Leu 140 , Asn
143, Tyr
165 form a substrate binding pocket.
With respect to catalysis, a well-ordered water was found between
in hNaa60. Chen et al. mutated these residues to alanine and
phenylalanine to confirm the role of theese residues in catalysis.
When these residues were mutated, catalytic activity of the protein
was abolished.
VI. References
Aksnes, Henriette, Van Damme, Petra, Gev.
2014. An Organelle Na-Acetyltransferase, Naa60, Acetylates
Cytosolic N Terminal of Transmembrane Proteins and Maintains Golgi
Integrity. Cell Reports10: 1362 - 1374.
Chen, Ji-Yun, Liang, Liu, Chun-Ling, Cao, Li,
Mein-Jun, Tan, Kemin, Yang, Xiaohan, Yun, Cai-Hong .2016.
Structure and function of human Naa60 (NatF), a Golgi-localized
bi-functional acetyltransferase. Sci. Rep. 6, 31425; doi:
10.1038/srep31425(2016).
Stove, Svein Isungset, Magin, Robert S.,
Foyn, Havard, Erik Haug, Bengt, Marmorsxtein, Ronen, Arnesen,
Thomas .2016. Crystal Structure of the Golgi-Associated Human
Na-acetyltransferase 60 Reveals the Molecular Determinants for the
Substrate-Specific Acetylation . Cell Reports Structure
24:1044-1056.
Back to Top