Hemoglobin Transport
Protein
Jordan Levin '19
Contents:
I. Introduction
Hemoglobin is an oxygen transport protein found in
vertebrates. The transport protein is crucial for life of multi
cellular organisms that require a constant availability of oxygen
for cellular respiration. Hemoglobin is found in red blood cells
binding to four oxygen molecules in the lungs and transports them to
the tissues.
Hemoglobin is not only an oxygen binding protein, it plays
a key role in the respiratory pathway which includes binding to
carbon dioxide once oxygen is released. The carbon dioxide gets
transported from the tissues to the lungs, then exhaled out of the
body. Hemoglobin can also bind to carbon monoxide and nitric
oxide.
II. General Structure
The human hemoglobin protein is made up of four subunits, two
identical alpha subunits ( A and
C , 141 residues) and two identical beta subunits (
B and D , 146
residues).
Each subunit binds to a
which contains an iron ion in the center and is
responsible for binding to oxygen.
III. Oxygen Binding
Although there are multiple carbon ritch residues that bind to the heme by Van der Waals interations,
histidine 87 directly attaches to the central iron ion
in the heme group by a coordinate covalent bond
. Electrostatic interactions explain polarity of heme binding pocket
. The binding pocket and the heme group go through a conformational
change when oxygen binds to the heme. The heme has a domed
configuration when it is deoxygenated but when oxygen is present
it adopts a planar
configuration.
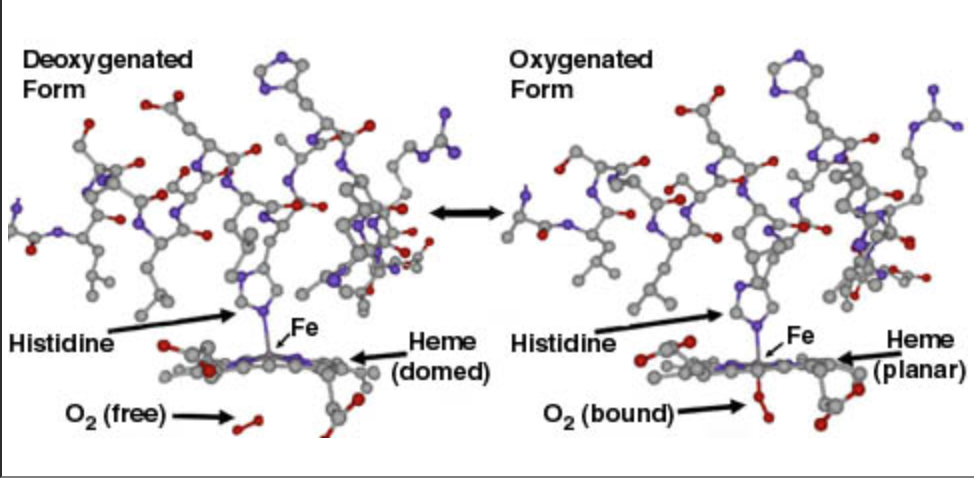 These two configurations are known as the ‘T’ or tense
structure where the protein has a low affinity for oxygen and
the ‘R’ or relaxed structure where oxygen binds with higher
affinity. Hemoglobin oxygen binding is classified as
cooperative binding meaning that once one oxygen is bound to a
heme, the other three hemes have a higher affinity for oxygen.
There is a conformational change throughout the protein making
the iron ion in the heme group more accessible to
oxygen. The change from ‘T’ to ‘R’ shifts the alpha
and beta F
helices
one angstrom and the beta E helix
two angstroms, opening the oxygen binding pockets.
Specifically, valine 67 on the beta E helices, which blocks oxygen
binding in the 'T' state, is moved to increase oxygen
affinity.
These two configurations are known as the ‘T’ or tense
structure where the protein has a low affinity for oxygen and
the ‘R’ or relaxed structure where oxygen binds with higher
affinity. Hemoglobin oxygen binding is classified as
cooperative binding meaning that once one oxygen is bound to a
heme, the other three hemes have a higher affinity for oxygen.
There is a conformational change throughout the protein making
the iron ion in the heme group more accessible to
oxygen. The change from ‘T’ to ‘R’ shifts the alpha
and beta F
helices
one angstrom and the beta E helix
two angstroms, opening the oxygen binding pockets.
Specifically, valine 67 on the beta E helices, which blocks oxygen
binding in the 'T' state, is moved to increase oxygen
affinity.
IV. Regulation
Once the oxygen is bound to the hemoglobin it is moved
via blood in the arteries. Regulation of hemoglobin occurs in
an acidic environment where allosteric inhibition triggers the
release of oxygen. The acidic environment is caused by
carbonic acid which is concentrated in high cellular
respiration areas. Carbon dioxide is produced as a byproduct
of cellular respiration and in the presence of water, produces
carbonic acid and hydrogen ions. The
Bohr effect explains that hemoglobin has a lower
affinity for oxygen when the pH is low and a higher affinity
for oxygen when the pH is high.
V. Sickle Cell Anemia
Sickle cell anemia is caused by a genetic point
mutation on residue 6 of both beta subunits. Wild type
hemoglobin has a glutamate on position 6 but the mutated
hemoglobin has a valine. The residue change from a polar
sidechain to non polar is the basis of sickle cell
aggregation. The nonpolar valine can form Van der Waals
interactions with either a luecine on residue 88 or
phenylalanine on residue 85 of an adjacent deoxygenated beta
subunit of
hemoglobin.
This same interaction can form long polymer fibers
within a red blood cell creating the sickle cell shape.
These mutated red blood cells can easily clog capillaries
resulting in painful swelling and reduced blood
circulation.
VI. References
Fermi, G., Perutz, M. F., Shaanan, B. 1983. The Crystal Structure of Human Deoxyhaemoglobin at 1.74 A Resolution. J. Mol. Biol., 159-174.
Harrington, DJ., Adachi, K., Royer, WE Jr. 1997. The High Resolution Crystal Structure of Deoxyhemoglobin S. J Mol Biol., 398-407.
Thomas, Caroline., Lumb, Andrew B. 2012. Physiology of Haemoglobin. Continuing Education in Anesthesia Critical Care and Pain. Vol 12, Issue 5.
Image 1: Traverso, Matt. 2004. Conformational Changes Upon Binding of Oxygen. Washington University in St Louis.
Image 2: Thomas, Caroline., Lumb, Andrew B. 2012. Physiology of Haemoglobin. Continuing Education in Anesthesia Critical Care and Pain. Vol 12, Issue 5.
Image 3: Bohr Effect Oxygen Release Explained: Healthy Vs Sick People. Normal Breathing.
Image 4: Mukerji, Ishita. Understanding Fiber Formation. About Sickle Cell Disease.
Image 5: Dr. Elebute, Modupe. 2016. Sickle Cell Disease. The London Physician.
Back to Top