E. coli Q-beta
Replicase Bacteriophage Replication
Lizzy Apunda '22 and Isabel Jaffer '22
Contents:
I.Introduction
Bacteriophage QB is a member of the leviviridae
family
(Brown, Fiedler, & Finn, 2009). It is a small virus that is
about 25nm thick and is a coliphage with an RNA that is 4217
nucleotides long. QB has 20 faces each composed of six subunits and
12 vertices each composed of 5 subunits. Members of the leviviridae
family form icosahedral capsids from 180 coat protein subunits
around a 4.2 kb sense-strand RNA genome (Singleton et at., 2018).
Each of these coat proteins (capsomers) has about 132 residues of
amino acids.
Bacteriophage QB is a positive strand RNA virus. Positive
strand RNA viruses have genomes that are functional mRNAs (Payne,
2017). For instance, QBís genome codes for 4 proteins: A1, A2, CP
and qb replicase. QB has other proteins like the B-subunit of a
replicase, the maturation protein A2 and a minor protein A1
(Singleton et al., 2018). The penetration of the virus into a host
cell is quickly followed by translation to produce RdRps and other
viral proteins that are required for the production of more viral
RNAs. QB ssRNA adsorb to bacterial sex pili proteins and infect.
Like other RNA viruses, QB replicates its genome by utilizing
virally encoded RNA polymerase (RdRp) (Payne, 2017). The genome is
used as the template for the synthesis of other RNA strands. Upon
infection, the B-subunit interacts with host proteins to form a
complex. The complex contains RNA-helicases to unwind DNA and NTPases
that are useful for polymerization. Once the complex forms, the
transcription of the genome, a copy of the genome, and mRNAs begin
(Payne, 2017). Phage MS2 has the same genome as QB.
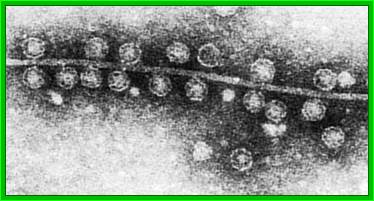
Figure 1: Q-beta
phage particles under a microscope
II. Mechanism/Infection
Upon infection, the bacteriophage
binds in large numbers to E.coli cells that have
the F-pili (Grumet et al., 1987). The tubular sex
pili are composed of oligomeric protein known as
Pilin, which allow the empty genome to escape
leaving behind an empty capsid (Grumet et al.,
1987). At this point, the B-subunit recruits host
translation factors EF-Tu and EF-Ts and ribosomal
protein S1 to form a QB replicase holoenzyme that
drives transcription.
The viral genome acts as an mRNA that hijacks the
hostís translation machinery to produce coat and
replicase proteins. Even though the viral genome
is linear, it contains hair loops even at the 5í
and 3íends. Hence, the helicases in the complex
unwind DNA, which makes it easier to transcribe.
One of the first replicase proteins
to be transcribed include the RNA dependent
Polymerases (RdRPs). The RdRPs contain the
catalytic machinery necessary for polymerization,
initiation and termination (Gytz et al., 2015).
Initiation and termination require the recruitment
of host proteins. RdRps of a number of positive
sense RNA viruses oligomerize and have a
stimulatory effect on RNA synthesis and viral
viability (Gytz et al., 2015).
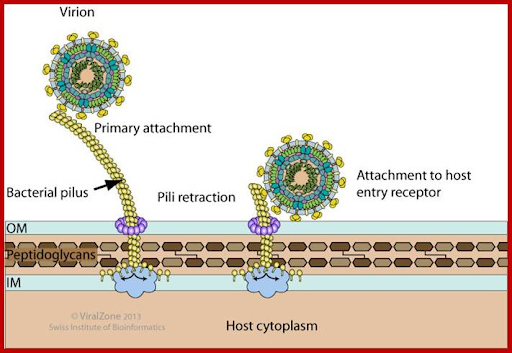
Figure 2A: An image
illustrating QB phage attachment to
host entry receptor
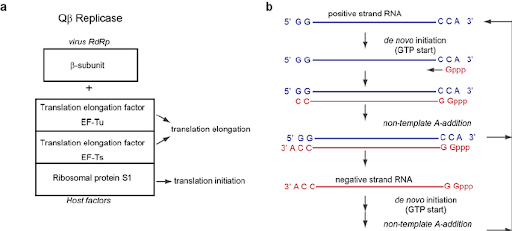
Figure
2B:Composition of
QB replicase and
replication cycle of QB
RNA. ( a ) Composition of
QB replicase. QB replicase
consists of the
virus-encoded
RNA-dependent RNA
polymerase (B -subunit),
and three host translation
factors: elongation factor
(EF)-Tu, EF-Ts, and
ribosomal protein S1; ( B
) Replication cycle of QB
RNA. QB virus has a single
positive strand RNA. The
positive and negative
strand QB RNAs both have
5'-GG and CCA-3'
sequences. A-3' does not
serve as a template
nucleoside, and is added
at the terminal stage of
RNA synthesis without a
nucleic acid template
(Tomita, 2014).
III. General
Structure
When E.
coli is infected by
Bacteriophage QB, a
core complex
consisting of the
and host elongation
factors (EF-Tu and
EF-T) is formed. The
EF-Tu and EF-T
elongation factors, as
well as ribosomal
proteins, work in
conjunction with the B
subunit. The B subunit
is the catalytic
domain for
RNA-dependent RNA
polymerization. It
consists of three
domains: the palm
(disordered), the
, and the fingers.
The thumb
domain contains three
segments.
is alpha-helical and precedes the fingers domain, while
comes after the palm domain.
is known as the C-terminal segment. It comprises the three-stranded
B-sheet at the tip of
the thumb domain.
The fingers domain is
comprised of
four-stranded,
antiparallel B-sheets.
It contains three
segments:
, which precedes the
palm domain,
, which is inserted in
the palm domain
between motifs A and
B, and
, which is a single
alpha-helix and is
also known as the
T-helix.
Other RdRPs
have an F-motif in the
N-terminal of their
sequences where the
thumb and fingers
domain are connected
(Kidmose et al.,
2010). This motif may
be responsible for
template unwinding. In
the Beta subunit, the
function of the
F-motif is fulfilled
by highly
in the fingers domain with assistance from the
that connect the thumb and the fingers
.
The fingers domain and
thumb domain are
connected by the
Ďbridgeí region which
flank the
in the fingers domain (Kidmose et al., 2010). The bridge region
consists of two
flexible segments
.
The bridge is
responsible for
preventing the unwound
template and product
strands from
reannealing by
limiting the cleft
above the catalytic
center. It is also
located in the
periphery of the
predicted path of the
duplex, acting as a
strand
separator.(Residues
520-532 are part of
the bridge and are
disordered) .
The B-subunit
has the ability to
interact with
substrates and
products. First, the
template likely enters
through the channel of
the fingers domain
. This template is
bound by which are
contained in a large
loop. The template is
also bound by one side
of the four-stranded
antiparallel Beta
sheet, which is
located in the fingers
domain. The presumed
substrate entrance
channel for NTP is
located between and
from the palm domain
along with and in the
fingers domain .
The substrate channel
through which NTP
enters is located in
motif D and A from the
palm domain along with
segments in the
fingers domain
. Five conserved
lysine and arginine
side chains, which are
located on either side
of the substrate
channel, coordinate
the incoming NTP .
At the
catalytic site, Asp274
(Motif A) and
Asp359-360 (Motif C)
have the ability to
synchronize two Mg2+
ions that can mediate
catalysis
. Lys214 and Arg220,
which are tightly
conserved, also have
the ability to form
electrostatic
interactions with the
NTP phosphates
IV. QB-RNA
replication Enzyme
The
replication enzyme
is known as the QB
replicase
holoenzyme. This
enzyme consists of
four proteins: two
B-subunits from
the phage,
ribosomal protein
S1, EF-Tu and
Ef-Tís.
Intermolecular
contacts in the
monomeric and
dimeric Qbeta
replicase core
complexes involve
the interface
between the two
Beta subunits
within the
putative dimer of
the core enzyme.
The
contribute to the dimer interface. The elongation factors and the
protein are
encoded by the
host. The
B-subunits
interact via a
symmetric network
of salt bridges
(Gytz et al.,
2015). Residues
Arg132 and Arg133
of each B-subunit
form
with the opposing
B-subunitís
Asp348. This dimer
is also stabilized
by
.
The most
significant
interactions
occur between
EF-Tu domain 2
and the fingers
domain, which
includes the
insertion of the
T-helix into the
binding pocket
for
CCA-aminoacyl
group of aa-tRNA
bound to EF-Tu:
GTP ( Kidmose et
al., 2010).
Tyrosine forms
hydrophobic
interactions and
a hydrogen bond
with glutamic
acid
, while arginine
residues of the
Beta subunit
form a salt
bridge
with EF-Tu
glutamine
residues.
The
B-subunit and
EF-Tu form an
interface that
is stabilized
by a molecule
of of the
precipitant
pentaerythritol
propoxylate
(PEP) (Kidmose
et al., 2010).
This results
in increased
hydrophobic
interactions
of the
neighboring
EF-Tu Phe 261
with a
hydrophobic
cluster of six
phenylalanine
and one
leucine side
chains from
the fingers
domain
.
VI.
References
Brown,
S., Fiedler,
J., &
Finn, M.
(2009).
Assembly of
Hybrid
Bacteriophage
Q? Virus-like
Particles.
Biochemistry,
48(47),
11155-11157.
doi:
10.1021/bi901306p
Grumet,
R., Sanford,
J. C., &
Johnston, S.
A. (1987).
Pathogen-derived
resistance to
viral
infection
using a
negative
regulatory
molecule.
Virology,
161(2),
561-569.
Gytz,
H., Mohr, D.,
Seweryn, P.,
Yoshimura, Y.,
Kutlubaeva,
Z., Dolman,
F., ... &
Knudsen, C. R.
(2015).
Structural
basis for
RNA-genome
recognition
during
bacteriophage
QB
replication.
Nucleic acids
research,
43(22),
10893-10906.
Kidmose,
Rune T.,
Vasiliev,
Nikita N.,
Chetverin,
Alexander B.,
Andersen,
Gregers Rom,
and Knudsen,
Charlotte R.
(2010).
Structure of
the QB
Replicase, an
RNA-dependent
RNA polymerase
consisting of
viral and host
proteins.
PNAS, 107(24),
10884-10889.
Payne,
S. Viruses.
2017
Singleton, R. L., Sanders, C. A.,
Jones, K., Thorington, B., Egbo, T., Coats, M. T., &
Waffo, A. B. (2018). Function of the RNA Coliphage QB
Proteins in Medical In Vitro Evolution. Methods and
protocols, 1(2), 18.
Tomita, K.
(2014). Structures and Functions of Q? Replicase:
Translation Factors beyond Protein Synthesis.
International Journal Of Molecular Sciences, 15(9),
15552-15570. doi: 10.3390/ijms150915552
Back
to Top