Structure of UvrA nucleotide
excision repair in complex with modified DNA in T. maritama
Amir Brivanlou '21 Ishan Mirchandani '20
Contents:
I. Introduction
Inaccuracy in DNA replication and exogenous damages can have
severely deleterious effects on an organism. Exposure to UV radiation can lead to a wide variety of DNA damages
from thymine dimers, to bulky adducts on a base. Many DNA repair mechanisms recognize specific forms of DNA damage,
however, the nucleotide excision repair (NER) mechanism recognizes a vast array of distortions in the DNA backbone. This promiscuity
allows the
NER to recognize and correct a wide variety of DNA damage within the cell. The NER in bacteria and archaea is carried
out by four main proteins, UvRA, UvRB, UvRC, and UvRD. First, two different UvRA proteins recognize sites both upstream
and downstream of DNA the damage site. UvRB then binds UvRA and confirms that the damage is present. UvRA disassociates
from UvRB and the UvRC protein is recruited and cuts the backbone 4 or 5 bases 3' to the damaged base, and about 8 sites 5'
from the damaged area. The UvRD exonuclease removes the cleaved strand, and DNA Pol I repolymerizes the removed DNA. DNA ligase
then reforms the phosphodiester bonds in the backbone. It is unclear whether UvRA recognizes the DNA alone, or if UvRB also plays
a part in the damage recognition.
UvRA is the first protein to recognize the sites of DNA damage.UvRA itself is a
dimeric protein that belongs to the ATP cassette binding (ABC) proteins which, interestingly, also contains
the bacterial MutS protein. UvRA itself contains two ATP binding regions whose structure will be characterized.
The mechanism by which UvRA recognizes damaged DNA is largely unknown despite being a well studied biochemical pathway.
The crystal structure of UvRA is from Thermotaga maritima,
a gram-negative bacteria like E. coli.
The protein was crystallized bound to two ADP molecules, and each dimer is composed of 6 subunits. By the study of this crystal structure,
we hope to point out some key interactions between the protein and DNA, as well as between UvRA and UvRB.
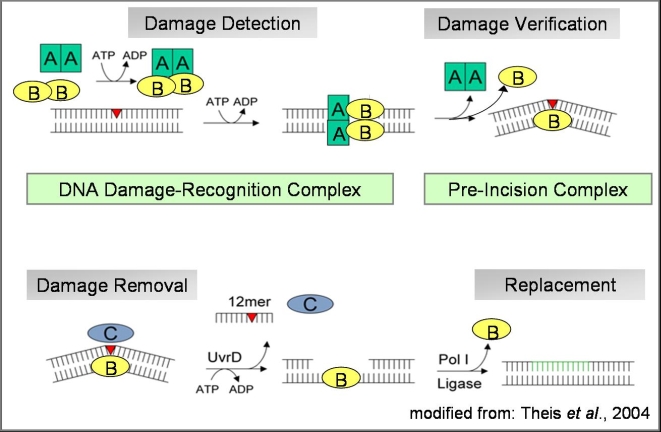 Figure 1.
Figure 1.
II. General Structure
The TM UvRA is composed of 6 individual subunits, two signature motifs, two ATP binding domains,
a DNA binding domain, and a UvRB binding domain. As we will see, these subunits contribute to multiple facets of protein function,
and their names only describe a part of their function. The core of the protein is centered around the two ATP binding domains. ATP binding
domain I is composed of 5 a-helices , and a core of B-sheets . It is also crucial for
maintaining contact in the dimeric form of the protein as the point of intersection of the two dimers is the ATP domain I .
ATP binding domain I is linked to the second ATP binding domain by a
structural linker . ATP binding domain II ,
much
like its counterpart binds one molecule of ATP. Two main a-helices flank a central group of sheets.
When ATP or ADP is bound, allosteric effects from the binding increases the affinity for damaged DNA.The signature Motifs I and II have varied functions.
Both signature domains form crucial parts of the ATP binding active sites, in fact, residues in
each signature motif, and each ATP-binding domain bind ADP/ATP in the core of the protein. Signature motif I
is a central component of the protein ,it is bound to ATP domain I,
the UvRB binding domain , and DNA binding domain. It is composed of
5 a-helices, a few sheets, and linker motifs .
Signature motif II plays an important role in DNA binding and stabilization, it is only linked to ATP binding domain II . It too is mostly composed
of a-helices , and residues 728-734 protrude from signature domain II and help to stabilize the interactions with DNA . When UvrA is dimerized, the UvrB
binding domain from one monomer and the signature motif II from the other bind UvrB. This interaction will be detailed later, however, it is important
to note that the UvRB binding domain is a small subunit composed primarily of two a-helices flanking a central B-sheet motif. Finally, the DNA
binding motif , which is linked to signature motif II , is primarily composed of 3 a-helices . The DNA binding motif is the only domain to move after ATP
binding, it acts as a clamp, moving downwards to increase the strength of UvRA-DNA binding.
III. DNA Binding
Tm-UvrA binds DNA in a non-sequence specific manner (Movie 1), and only interacts with the DNA backbone.
UvrA binds to DNA with lesions, which are indicated by a characteristic stretch and shear on the affected nucleotides. Because UvRA makes no specific backbone interactions, it is capable or recognizing several forms of DNA damage, increasing its utility.
Eight residues in signature domain II interact with the backbone of four nucleotides.
The other residue which has been found to be definitively involved with DNA binding is Lys660 of the ATP-binding domain II .
Despite its distance from the backbone it is integral in DNA binding, as mutants lacking Lys660 are found to have a much lower binding affinity.
The DNA binding domain is located just above the midpoint of bound DNA, and is believed to clamp down on the DNA.
Although no specific residue-nucleotide contacts have been found, the deletion of the DNA binding domain leads to significantly lower binding affinity with DNA.
IV. ATP Binding
UvRA can bind two molecules of ATP. Upon ATP binding, the protein undergoes a slight structural change.
The DNA binding motif swings down increasing the strength of UvRA-DNA interactions.
Additionally, the signature motif II pull inwards,
creating an open dimer conformation. The signature motif II plays a crucial role in DNA binding, and the open complex is more
conducive to DNA recognition and binding. UvRA contains two active sites for binding ATP. The first active site is formed at the
intersection of the ATP binding domain I , and signature motif II .
More specifically, Serine 807 of signature motif II and Lysine 37 of ATP binding domain I, are responsible for binding and stabilizing ATP/ADP.
The exact mechanism of this interaction is unknown,
however, it is thought that interactions occur primarily with the pyrophosphates of the ATP/ADP molecule. The second active site is
formed by ATP binding domain II and signature motif I. Much like the other active site,
a serine, and a lysine are responsible for the
binding and stabilization of the ATP/ADP molecule in the binding site. The exact function of ATP to UvRA is still an open problem. Studies
have shown that ATP binding increases affinity for DNA with lesions and increases the overall strength of binding with DNA, however, the mechanism
by which these changes occur remains to be elucidated.
V. UvRB Binding
Because UvrA is a dimeric protein, it can associate with two UvrB monomers to form a
UvrA2–UvrB2 complex, with each UvrB protein associating with opposite sides of the UvrA dimer. To visualize the UvRA-B interactions, we
are swithing over to a different organism's UvRA-B complex. This complex was crystalized from Geobacillus stearothermophilus, a gram positive bacteria.
The UvrA2–UvrB2 structure is curved, with the central UvrA dimer composing the bottom of the trough. UvrB contacts UvrA at signature domain II ,
and the UvrB binding domain. Glu295 and Glu299 in UvrB , which contact signature domain II of UvrA, are central to association with UvrA,
though the nature of these interactions are not known . These two residues do appear to fit nicely within a pocket in the signature domain II . When either Glu295 or Glu299 were knocked out, the association between
UvRA and UvRB decreased significantly. The Val204 residue in the UvrB-binding domain of UvrA forms a hydrogen bond with Asp198 in UvrB .
These two interactions form the basis of UvrB association on each side of the UvrA dimer .
VI. References
-
Jaciuk, Marcin, et al. “Structure of UvrA Nucleotide Excision Repair Protein in Complex with Modified DNA.” Nature Structural & Molecular Biology, vol. 18, no. 2, 2011, pp. 191–197., doi:10.1038/nsmb.1973.
-
Kisker, C., et al. “Prokaryotic Nucleotide Excision Repair.” Cold Spring Harbor Perspectives in Biology, vol. 5, no. 3, 2013, doi:10.1101/cshperspect.a012591.
-
Pakotiprapha, Danaya, et al. “Structure and Mechanism of the UvrA–UvrB DNA Damage Sensor.” Nature Structural & Molecular Biology, vol. 19, no. 3, 2012, pp. 291–298., doi:10.1038/nsmb.2240.
-
(Figure 1) Prokaryotic Nucleotide Excision Repair.” Nucleotide Excision Repair, www.virchow-zentrum.uni-wuerzburg.de/kiskerlab/pro_ner.html#proNER.