omega-aminotransferase is a class
III aminotransferase that has a substrate specificity for
(S)-a-Methylbenzylamine (MBA), beta-alanine, 4 aminobutyrate and
pyruvate. Aminotransferases catalyze the transfer of an amino group from
an amino to a keto acid using pyridoxal phosphate (PLP) as a cofactor.
During transamination, aminotransferases bind to PLP to form a Schiff
base with a lysine in their active site resulting in an internal
aldimine. PLP subsequently forms an external aldimine with the amino
donor. Hydrolysis of the resulting ketamine leads to keto product, but
leaves PMP which must be regenerated to PLP. In a dehydration reaction,
the keto donor forms a Schiff base with PMP. Lysine dehydrogenates and
then displaces the transaminated ketodonor to regenerate the internal
aldimine PLP from the external aldimine.
Scheme 1. General aminotransferase reaction mechanism using the PLP
cofactor.
The majority of aminotransferase classes catalyze the transamination
of alpha amino acids (classes I,II, IV and V). However, class III
aminotransferases catalyze transamination of substrates with amines at
omega positions, and are known as omega-aminotransferases. This
reaction is more challenging to catalyze, but many amino transferase
reactions with industrial applications lack an alpha carboxyl group,
so there is much interest in class III omega-aminotransferases which
are able to do these reactions.
Pseudomonas aeruginosa omega-aminotransferase is a
with two catalytic dimers. It has a molecular weight of 200
kDa, and each monomer has a molecular weight of 50 kDa. Two
at the interface of the two dimers stabilize the tetramer.
Each calcium ion is coordinated by the carboxyl groups of Asp180 of
the two adjacent subunits as well as four water molecules
. Four evenly spaced
, one for each subunit, sit in pockets formed at the interface of the
noncatalytic dimers. Each of the chloride ions coordinates with
Phe173, Phe322, Ser323, and Met172
.
Omega aminotransferase fits into the class of type I PLP fold
enzymes which consist of a
.
The small domain comprises N- and C-termini of the polypeptide
chain. The
folds into an alpha/beta/alpha sandwich made up of a
seven-stranded beta sheet. The
is made up of two beta sheets; a four-stranded N-terminal
beta sheet (the last sheet is donated by C-terminus) and an
antiparallel beta sheet which is pinned between three alpha helices
on one side and the large domain. This interface between the large
and small domains composes the active site of the enzyme. The enzyme
contains four cofactor binding domains, at the bottom of each active
site. All bound
While PLP is the natural cofactor for P. aeruginosa omega-aminotransferase,
during crystallization of the enzyme PLP crystalized outside of the
active site. However, PLP crystallized in the active site when it
was bound to the suicide inhibitor gabalucine, resulting in PXG. PXG
was subsequently used as a proxy for PLP in the analysis of active
site interactions with the cofactor.
III. PLP binding
When Pseudomonas aeruginosa omega-aminotransferase binds
, it induces a conformational change in its active site. The
(up to residue 36), which is disordered in the apoenzyme
(unbound) structure, becomes ordered in the holoenzyme (bound)
structure and occupies the position of the unwound helix.
The holoenzyme structure features bound cofactor, Schiff-base
PLP–Lys288, bound at the bottom of the active site. PLP binds
at the interface of the catalytic dimer, between the two
domains of a
, where the Lysine is in between beta strands 9 and 10. The
phosphate group of PLP makes hydrogen bonds to Gly120, Thr327,
Thr327, and Ser121
. The carboxyl group of Asp259 makes a hydrogen bond to the
pyridine-ring N atom of PLP
. Asp259 is stabilized by interactions with the imidazole
ring of His154
. The pyridine ring of PLP is sandwiched between the side
chains of Tyr153 and Val261, which lie perpendicular to the cofactor
ring
.
Cocrystallization with gabaculine, the irreversible GABA
aminotransferase inhibitor, allowed for the determination of the
inhibitor complex structure. The inhibitor-bound complex provides
valuable information about the active-site, the determinant of the
substrate specificity. Gabaculine is covalently bound to C4 of PLP
as the mCPP complex in all four monomers. It binds to the carboxyl
group of mCPP and makes hydrogen bonds to the side-chain O atom of
PA Gln421 and the side-chain N atoms of PA Trp61 and PA Arg414.
These three residues form a rigid
.
IV. Substrate Specificity
Class III omega-aminotransferases all catalyze transaminations at
omega carbon positions, but they differ in substrate specificity.
Pseudomonas aeruginosa omega-aminotransferase has a narrow substrate
specificity, accepting only beta alanine, 4-aminobutyrate and MBA as
amino donors, and catalyzes amino transfer to pyruvate.
Scheme 2. Amino and Keto donors 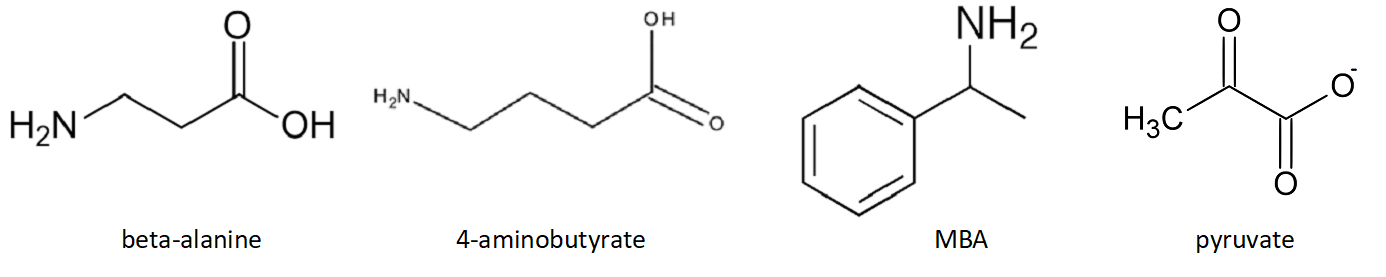
Sayer et al. (2013) used the suicide inhibitor of PLP
to interpret the mechanisms behinds Pseudomonas aeruginosa
omega-aminotransferase’s substrate selectivity. Unlike more flexible
aminotransferases, the enzyme has a
at a fixed distance from the cofactor, formed by the side chain O
atom of Gln421, and the side chain N atoms of Arg414 and Trp61. The
rigid structure of the omega aminotransferase’s active site limits
its substrate specificity, but makes it very active towards
beta-alanine.
When the native substrate, beta-alanine, is modeled in the
carboxylate binding site, the amino end is ideally positioned for
transamination. This is further favored by the positioning of
, which is involved in proton abstraction. When smaller
amino donors are bound at the carboxylate site, the amino group is
too far from PLP to form a Schiff base. Larger amino donors are not
able to occupy the active site as the side-chain of
sterically blocks any amino acid beyond the beta-carbon.
Understanding the mechanisms for substrate selectivity has
significant industrial applications. Omega aminotransferases are
promising tools for synthesizing medically-relevant enantiopure
compounds due to their broad substrate specificity, high
enantioselectivity, and high turnover number as well as no need for
cofactor regeneration.
Omega aminotransferases have been engineered to efficiently
produce optically pure amines and beta amino acids (Cho et al.,
2008; Shin et al., 2015). Further research has explored the
potential for omega aminotransferases to catalyze enantiopure ketone
synthesis, however, more work is needed to establish omega
aminotransferases as an efficient means to producing this class of
compounds (Han et al., 2019).
V. References
Cho, B. K., Park, H. Y., Seo, J. H., Kim, J., Kang, T. J., Lee, B. S., & Kim, B. G. (2008). Redesigning the substrate specificity of omega-aminotransferase for the kinetic resolution of aliphatic chiral amines. Biotechnology and bioengineering, 99(2), 275-284.
Han, S. W., & Shin, J. S. (2019). Activity Improvements of an Engineered omega-transaminase for Ketones Are Positively Correlated with Those for Cognate Amines. Biotechnology and Bioprocess Engineering, 24(1), 176-182.
Shin, G., Mathew, S., & Yun, H. (2015). Kinetic resolution of amines by (R)-selective omega-transaminase from Mycobacterium vanbaalenii. Journal of Industrial and Engineering Chemistry, 23, 128-133.
Back to Top