Human medium chain μ2 adaptin
subunit (AP50) of adaptor protein complex 2
Jo Bùi '22 and Isabelle Freeman '23
Contents:
I. Introduction
The human medium chain μ2 adaptin subunit
is a component of the clathrin-associated adaptor protein complex 2 (AP-2)*, a heterotetramer of 4 subunits:
,
,
, and
.
The complex functions in endocytosis, specifically cargo
selection and vesicle formation, and is activated by the
phospholipid component
. Its function consists of 3 main sequential activities: recruitment
to the plasma membrane, binding specific signals from cargo
transmembrane proteins, and anchoring the polymerization of clathrin
into a polyhedral vesicle coat.
The μ2 subunit is mainly involved in the second activity,
recognizing tyrosine-based
(x is any amino acid
and φ a bulky hydrophobic amino acid that can be Leu, Ile, Met, Phe, or Val)
. However, it is only accessible for signal binding when the complex
is in the unlocked(or activated) conformation, where the binding
sites are not occluded by other subunits.
*There have been no successful attempts to crystallize the
phosphorylated AP50 subunit in the unlocked conformation all displayed
open complexes are merely theoretical models.
II. General Structure
AP50, when complexed with CTLA-4, is an asymmetrical
heterodimer of 34.21 kDa, composed of 229 residues making up two
unique polypeptide chains.
The
is comprised of a N-terminal
domain that is tightly bound into the core (α and β2) and a
C-terminal domain (CTD) that swings out
from the core upon activation of the complex
. The two domains are connected by a linker that also changes
conformation upon activation.
μ2 CTD, the more active domain, is a nearly all β-sheet
sandwich structure that consists of 2 subdomains,
and
.
III. μ2 Active Sites
The linker connecting N- and C- terminal domains contains
(although it is referenced in other papers as Thr156), which is a
site of phosphorylation by AAK1 (α-appendage binding kinase) or
cyclin-G associated kinase (GAK. Thr375 phosphorylation
promotes cargo interactions with AP-2 in
vitro and in vivo.
Subdomain A of μ2 CTD contains the
for Yxxφ signals that is occluded by part of β2 subunit in the locked
conformation
Subdomain B contains a PI(4,5)P2 binding
site that is solvent exposed in both the locked and unlocked
conformations. It does not play a role in the PI(4,5)P2
binding-mediated recruitment of AP-2 to the membrane, however it
is important in AP-2 activation.
There are
on subdomain μ2, close to the Yxxφ
motif binding site. Though these site are accessible in the locked
state, they have a minor effect on AP-2 activity
.
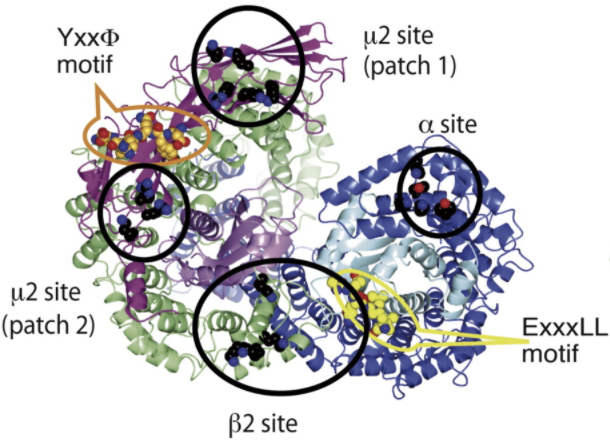 Figure 1
Figure 1
IV. AP-2 Unlocking and Stabilization
Video 1
N-terminal domain C-terminal
domain
The first step in AP-2 activation is its recruitment onto
the plasma membrane, primarily by binding of α or β2 to PI(4,5)P2. Once attached, the
and subsequent
binding of μ2 CTD to the high local concentration of PI(4,5)P2
causes AP-2 to adopt the open conformation.
The
, disordered in the locked conformation, can now fold into an
α-helix in the slot formed between N-μ2 and β2, again stabilizing
the open form. Further stabilization may be achieved through
phosphorylation of μ2 Thr375 by AAK1 or GAK.
The resulting electropositive environment leads to tighter
binding of AP-2 to cargo-containing membranes.
V. Binding Yxxφ signals
Binding of Yxxφ signals, the most important function of the
μ2 subunit, is high-affinity and high-specificity and happens only
when AP-2 is in the unlocked conformation. Yxxφ signals bind to μ2
CTD by β-augmentation to one edge of a sheet on subdomain A.
‘‘β-augmentation’’ refers to a peptide or protein binding
mode involving the incorporation of the bound ligand as one or
more additional strands of the β-sheet. Specificity is conferred
by two hydrophobic pockets that bind the Y and φ residues. The
aromatic side-chain of Y stacks with those of surrounding Trp,
Tyr, and Arg residues
, and its hydroxyl is hydrogen-bonded with a conserved Asp residue
.
The φ residue is bound in a hydrophobic pocket formed at
the juncture of the β-sheet sandwich
.
VI. References
Canagarajah, B. J., Ren, X., Bonifacino, J.
S., & Hurley, J. H. (2013). The clathrin adaptor complexes
as a paradigm for membrane-associated allostery. Protein
Science, 22(5), 517-529.
Follows, E. R., McPheat, J. C., Minshull,
C., Moore, N. C., Pauptit, R. A., Rowsell, S., ... & Abbott,
W. M. (2001). Study of the interaction of the medium chain μ2
subunit of the clathrin-associated adapter protein complex 2
with cytotoxic T-lymphocyte antigen 4 and CD28. Biochemical
Journal, 359(2), 427-434.
(Figure 1)(Video 1) Jackson, L. P., Kelly,
B. T., McCoy, A. J., Gaffry, T., James, L. C., Collins, B. M.,
... & Owen, D. J. (2010). A large-scale conformational
change couples membrane recruitment to cargo binding in the AP2
clathrin adaptor complex. Cell, 141(7), 1220-1229.
Olusanya,O.,Andrews, P. D., Swedlow, J. R.,
& Smythe, E. (2001). Phosphorylation of threonine 156 of the
μ2 subunit of the AP2 complex is essential for endocytosis in
vitro and in vivo. Current biology : CB, 11(11),
896–900. https://doi.org/10.1016/s0960-9822(01)00240-8
Owen, D. J., & Evans, P. R. (1998). A
structural explanation for the recognition of tyrosine-based
endocytotic signals. Science, 282(5392), 1327-1332.
Partlow, E. A., Baker, R. W., Beacham, G.
M., Chappie, J. S., Leschziner, A. E., & Hollopeter, G.
(2019). A structural mechanism for phosphorylation-dependent
inactivation of the AP2 complex. Elife, 8, e50003.
Back to Top