Dopamine β-hydroxylase
Kirollos Mikhaeel 22' and Trenton Scherger 23'
Contents:
I. Introduction
Dopamine β-hydroxylase (DBH) is a glycoprotein of the catecholamine
biosynthetic pathway that catalyzes the hydroxylation of dopamine to
norepinephrine (Scheme 1.). This reaction is executed using an O2
molecule as the oxygen source of the hydroxyl group added to the
β-carbon in dopamine. DBH has a molecular weight of 290 kDa containing
617 amino acids (Kapoor,
Abhijeet, et al., 2011).
DBH is found in both the central and peripheral nervous systems and
is the only source of norepinephrine and epinephrine in the body.
Improper balancing of dopamine to norepinephrine in the body is the
cause of many diseases including: hypertension, Alzheimer’s and ADHD (Vendelboe,
Trine V, et al., 2016).
DBH is a member of a class of copper-containing hydroxylases found in
eukaryotes which play a pivotal role in the biosynthesis of hormones
and neurotransmitters; those coppers are the the main components of
the active site of DBH that catalyzes the reaction.
CuM and CuH are
the two components of the active site; CuM
is involved in the O2 binding and is the site where
norepinephrine is hydroxylated, and CuH
is the site where electron transfer takes place (Vendelboe,
Trine V, et al., 2016).
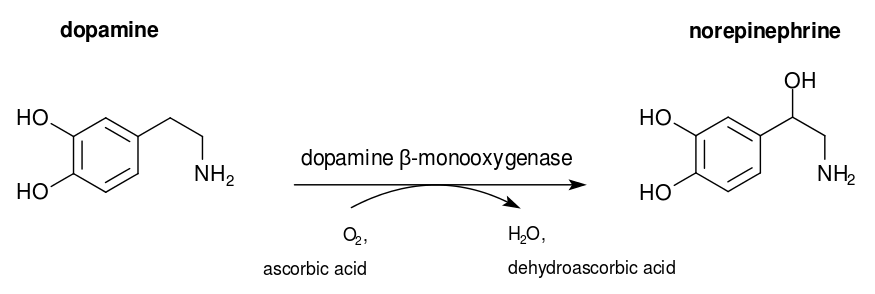
Scheme 1.
Hydroxylation of Dopamine into norepinephrine.
II. General Structure
DBH is a dimer that is composed of two main chains and 4 domains:
,
,
,
and
.
The DBH dimer is held together by
as well as
(Kapoor,
Abhijeet, et al., 2011). DBH requires 2 bound Cu atoms per
subunit to be active. The overall structure of the protein is
symmetric. It is mostly composed of β-sheets with some α-helices in
the middle (Vendelboe,
Trine V, et al., 2016).
From examination of the movement of electrons it was shown that the
closed complex (the one being examined here) is very
.
The "hot" colors represent atoms that are
“loose” in their arrangement, while "cool"
colors represent those that are “fixed”. Due to the abundance of deep
blue from this examination, this details that the closed structure of
DBH is very ordered and not subject to much change.
III. DOMON Domain
The DOMON domain (DD)
is an immunoglobulin-like- β-sandwich made up of
and a ligand-binding pocket. The core structure of the DD
folds up in a
consisting of one β-sheet with 5 antiparallel strands and another
β-sheet with 6 antiparallel β-strands in a β-sandwich. The C-terminal
sheet of the DD includes a
directly after the dimerization domain. The ligand binding pocket in
the DD is very
with several likely ligands such as ascorbate, dopamine, and
norepinephrine. Behind the ligand-binding pocket there is a metal
ion-binding site coordinated by
,
,
, and
.
Those 4 residues (as well as
and
in the vicinity) are conserved among DBH DDs
from different organisms. It is likely that either an alkali metal ion
or an alkaline earth metal ion binds to the ion-binding site (due to
the oxygen-rich environment). The DD is
linked to the C-terminal part of the protein via a
(C154 and C596). The C-terminal part of the
protein also contains
(Asn64 and Asn184); both could be built in chain
A, whereas in chain B glycan can only be built at Asn64 (Vendelboe,
Trine V, et al., 2016).
IV. Catalytic Domain
The catalytic core consists of two domains: the
where CuH binds and the
where CuM binds. The
N-terminal domain is characterized by its folding into a
consisting of 2 antiparallel β-sheets (4 and 5 β-strands in each case,
respectively) as well as 2 disulfide bridges that go between the
β-strands. One disulfide bridge is made between
and holds together the B4 and B6 β-strands. The other bridge is
between
that holds the B2 and B5 strands together. Another unique feature of
this domain is that glycosylation is observed at
, whereas the C-terminal domain does not share that, however, both
domains have glycosylation at Asn366. As for the C-terminal domain, it
also folds into a
but one β-sheet is antiparallel (4 β-strands) and the other is a mix
of antiparallel and parallel sheets (5 β-strands). Both sheets are
held together by a very hydrophobic interior
and disulfide bonds (
); B8-B9)(
; B2-B10) that go between β-sheets. The C-terminal domain is made up
of a 6 stranded sheet by the addition of β-strand residues (561-566)
from the C-terminal of DBH. This sixth β-strand is also stabilized by
a
(Vendelboe,
Trine V, et al., 2016).
The positioning of the domains in both chains (of the dimer) differs
as well. The CuH (N-terminal)
domain is moved
from the DOMON domain and
to the CuM domain in the
A chain, whereas the
is true in the B chain. In each chain there are two copper binding
sites making a total of 4 copper sites in DBH. ¾ of the sites are not
occupied, but the CuM
site in chain A is weakly occupied by a copper atom. Approximate
positions for the copper atoms in the CuH
domain is given by the
His262, His263 and His333 and for the
CuM domain,
are His412, His414 and Met487 (Vendelboe,
Trine V, et al., 2016).
V. Dimerization Domain
The dimerization domain (DiD)
consists of β-helices without any notable β-sheets. The DiD
is held together by
. The
are stabilized by
. The
is used to connect the structure to the Cu domains. The DiD
is stabilized by a C-terminal strand connected via two β-sheets into
the DOMON domain and the
CuM domain (Vendelboe,
Trine V, et al., 2016).
VI. References
- Kapoor, Abhijeet, et al. "Structural Insight of Dopamine
β-Hydroxylase, a Drug Target for Complex Traits, and Functional
Significance of Exonic Single Nucleotide Polymorphisms." PloS
One, Public Library of Science, 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3197665/.
- Vendelboe, Trine V, et al. "The Crystal Structure of Human
Dopamine β-Hydroxylase at 2.9 A Resolution." Science Advances,
American Association for the Advancement of Science, 8 Apr. 2016,
www.ncbi.nlm.nih.gov/pmc/articles/PMC4846438/.
- Bank, RCSB Protein Data. "4ZEL: Human Dopamine β-Hydroxylase." RCSB
PDB, www.rcsb.org/structure/4ZEL.
Back to Top