Mus musculus Myelin
Associated Glycoprotein (MAG)
Lara O'Callaghan '23 and Maggie Williard '23
Contents:
I. Introduction
The myelin sheath is essential for the nervous system beause it
insulates the axon and improves signal transmission by increasing the
speed and efficiency of the electrical impulse. Myelin-associated
glycoproteins (MAG) are specialized proteins that aid in the
stabilization, construction, regulation, and maintenance of the myelin
sheath. MAG acts as a bridge between the axon cell and the myelin
sheath to maintain the periaxonal diameter, known as myelin-axon
spacing (Fig. 1). MAG is a membrane glycoprotein and a member of the
Ig superfamily (immunoglobulin protein family). If MAG is compromised
via misfolding or autoimmune attacks, neurodegenerative disorders such
as multiple sclerosis can develop.
MAG proteins are dimers that interact with glycolipids to adhere to
the myelin sheath and bind to the axon to provide structure.
Additionally, MAG inhibits axon regeneration and controls myelin
formation. MAG consists of five Ig domains in a homodimeric
arrangement with membrane-proximal domains Ig5 and Ig4. MAG binds to
specialized lipids that reside on the axon membrane. MAG also has
trans-membrane non-structured tails that reach beyond the myelin to
interact with other proteins. Short linkers connect the MAG domains,
creating a rigid structure which allows for bidirectional interactions
between the myelin and the axon. MAG is post-translationally modified.
The protein structure of MAG was solved in Mus musculus, the common
house mouse.
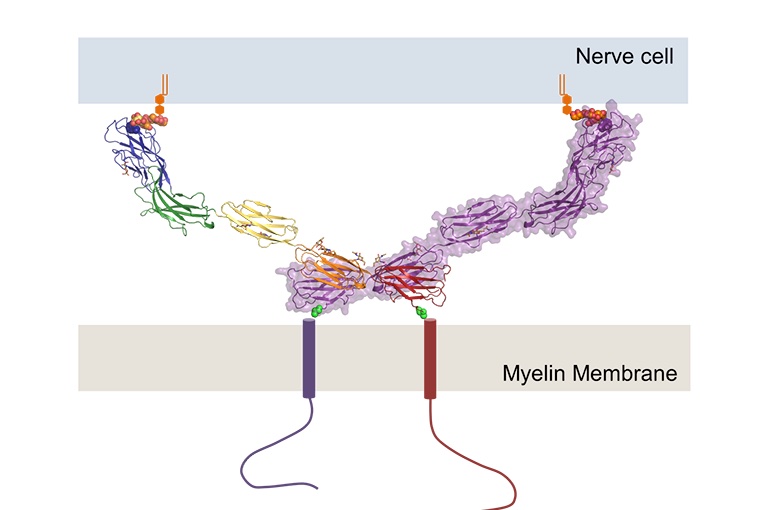
Figure 1. Structure of
Myelin-Associate Glycoprotein. (UMC Utrecht, 2016)
II. General Structure
Mag is a
; this means it is a protein composed of two chemically identical
polypeptide chains (Monomer 1 and Monomer
2) that mirror each other. Each
is an asymetric unit composed of five domains labled Ig1,
Ig2, Ig3,
Ig4 and Ig5.
. MAG is primarily composed of
but it also contains several
alpha helical structures. The Ig4 and Ig5 domains link the two
monomers together to form the homodimer; their binding forms two
equivalent hemi-interfaces that are largely hydrophobic and
hydrophilic. The Ig1 and Ig2 domains create the biggest interface
where Ig2 loops at the N-terminal side to interact with Ig1's A2-B
side. However, Ig3, Ig4, and Ig5 are only formed in a head-to-tail
manner at loops in the
head and
tail sides of the Ig domains.
III. Specific Structures and Binding Interactions
Each Ig domain is joined by
, creating a rigid structure that enables MAG to connect the myelin
and the axon. This allows MAG to moderate communication between the
membranes. The Ig1 domain's N-terminal has a V-type Ig fold; this is
consistent with other Siglec proteins. Meanwhile, the Ig3 and Ig4
domains are C2-type. The Ig2 and Ig5
domains have a C1-type Ig fold
.
MAG has an inter-domain disulfide bond between Ig1 and Ig2. MAG is
post-translationally modified at many sites, containing seven
. More specifically, five of these disulfide
bridges are cannonical for the Ig domains. An inter-domain
disulfide is formed between Ig1 and Ig2 by cysteines 37 and 165.
Another intra-domain disulfide in Ig5 is formed by cysteines 421 and
430. MAG contains a plethora of
whose binding can cause conformational changes, affecting the overall
structure. Ig1 plays an essential role in the recognition of ligands.
Mag also contains 6 strong covalent bonds. Additionally, MAG goes
under an N-linked glycosylation at the dimerization interface, which
acts as a post-translational modification. MAG has eight
sites. As the body develops, MAG glycosylation changes, and
myelination deficiencies are correlated with abnormal glycosylation.
IV. MAG Mutagenesis
Mutations in MAG can result in a plethora of aberrant functions.
Mutations can often cause MAG misfolding or anti-MAG autoimmunity.
This can have negative effects such as demyelination and
neurodegenerative disorders. Myelin deficiency in the CNS and PNS is
the result of the mutation of a gene that links signal transduction to
RNA metabolism. The mutation of
, which makes contact with sialic acid, on MAG reduces the potency of
the inhibitory activity of MAG.
VI. References
Goodsell DS. 2020 Jul 1. Myelin-associated
Glycoprotein. RCSB PDB. doi:10.2210/rcsb_pdb/mom_2020_7.
Lossos A, Elazar N, Lerer I, Schueler-Furman
O, Fellig Y, Glick B, Zimmerman B-E, Azulay H, Dotan S, Goldberg
S, et al. 2015. Myelin-associated glycoprotein gene mutation
causes Pelizaeus-Merzbacher disease-like disorder. Brain.
138(9):2521-2536.
Pronker MF, Lemstra S, Snijder J, Heck AJR,
Thies-Weesie DME, Pasterkamp RJ, Janssen BJC. 2016. Structural
basis of myelin-associated glycoprotein adhesion and signalling. Nature
Communications. 7(1):13584. doi:10.1038/ncomms13564.
Quarles RH. 2007. Myelin-associated
glycoprotein (MAG): past, present and beyond. Journal of
Neurochemistry. 100(6):1431-1448.
doi:10.1111/j.1471-4159.2006.04319.x.
UMC Utrecht. Structure of protein connecting
neuron and myelin clarified (December 6, 2016).
Vinson M, Strijbos PJLM, Rowles A, Facci L,
Moore SE, Simmons DL, Walsh FS. 2001. Myelin-associated
Glycoprotein Interacts with Ganglioside GT1b: A MECHANISM FOR
NEURITE OUTGROWTH INHIBITION*. Journal of Biological
Chemistry. 276(23):20280-20285. doi:10.1074/jbc.M100345200.
Yang LJ, Zeller CB, Shaper NL, Kiso M,
Hasegawa A, Shapiro RE, Schnaar RL. 1996. Gangliosides are
neuronal ligands for myelin-associated glycoprotein.Proc Natl
Acad Sci USA. 93 (2):814. doi:10.1073/pnas.93.2.814.
Back to Top