Homo sapien
brain-type creatine kinase
Sydney Buchman '24 and Kod McCune '24
Contents:
I. Introduction
Homo sapiens brain-type creatine kinase is a transferase
enzyme and belongs to the phosphagen kinase superfamily, and is the
only phosphagen located in vertebrate species.1,2 The
kinase is vital for cellular metabolism, and is found in high
quantities in the brain and retina of the body. Specifically, the
kinase is responsible for the reversible phosphoryl reaction of ATP
and creatine. The kinase takes the gamma phosphate group off a bound
ATP molecule and transfers to creatine - creating phosphorylated
creatine (PCr).1,3 This allows the body to store
phosphates on creatine, which prevents excessive buildup of ATP.
Such a buildup would inhibit glycolysis and the production of ATP.
When in need of ATP, the kinase can then work the reverse reaction
and transfer the phosphate back onto ADP, thus producing ATP for
cellular activity. The interplay between these two molecules is
crucial for energy transport and maintaining energy levels
throughout key organs of the body.3
Brain-type creatine kinase provides the necessary ATP
for brain function and brain energy metabolism. It has been found
that the kinase associates with ATPases, and regulates ion
transport crucial for brain functionality.1,4 Due to
its importance, deficiencies in creatine kinase levels have been
linked to severe neurodegenerative diseases.1,4,5

Figure 1: The reaction scheme of
the creatine phosphoryl transfer reaction. The phosphate group of
ATP is transferred onto creatine, yielding phosphocreatine and ADP
as products. The arrow indicates that this reaction is reversible,
which is an important feature for bodily function.6
The brain type creatine kinase (hBB-CK) is formed from two
isoenzymes, creating a
Each monomer within the homodimer is capable of binding to creatine
and ADP to perform the phosphoryl transfer reaction. Furthermore,
the dimer contains a N-Terminal alpha
helical domain, spanning residues 1-100, and a large C-terminal
alpha/beta domain present from residues
1,5 These two distinct domains are connected
with a linker region that comprises
residues
These pieces form the overall base structure of the homodimer protein.2
Additionally, residues Asp-54 and Arg-148
act as
interface residues, which produce salt bridges and
hydrogen bonding to increase the connectivity between the monomers.1,5
The active site of hBB-CK consists of multiple
residues. These include Arg132, Arg130, His191, Glu232,
Arg236, Cys283, Arg292, and His296. Residues His66, Ile 69, Val72,
on the short loop, and Val325 and Asp326 on the long loop are also
included.1
III. Nucleotide Bonding Interactions
When bound to the
(in order to facilitate the phosphoryl reaction), the
monomers of hBB-CK undergo a conformational change, causing the
enzyme to appear asymmetric.1,2 The bound monomer
assumes a
with the other unliganded protomer retaining an open
conformation. The closed form is characterized by the loops of
residues 60-70 and 323-332
changing position, where they can be seen moving into the active
site of the kinase.1 This has the side effect of
moving hydrophobic residues Ile69 and Val325 adjacent to the
methyl groups of creatine.
The ADP-Mg2+ complex binds to
the active site through a variety of interactions. The ribose
of the ADP participates in multiple
Water molecule are responsible for linking the adenosine with
the carboxylate group of Asp335, as well as the main chain
carbonyl oxygens of both Arg292 and Ile188.1 His191
forms a hydrogen bond with the 2'-hydroxyl group of
the ribose, and also the main-chain nitrogen of Gly294.1
The phosphates of the ADP interact
with the phosphate binding pocket, consisting of residues
Arg130, Arg132, Arg236, Arg292, and Arg320. The
partake in extensive hydrogen bonding with the oxygens
of the phosphate. Specifically, the N-eta-1 on Arg132 and
the N-epsilon on Arg292 create monodentate interactions with
the beta-phosphate. The Arg130 bonds with the beta-phosphate
and the ring-oxygen of the ribose. Arg236 forms a hydrogen
bond with the beta-phosphate, and Arg320 with the
alpha-phosphate.1 The last two residues (Arg236
and Arg320) form additional hydrogen bonds with nitrate ions
incorporated in the transition state of the phosphoryl
reaction.1 Mg2+ is also present in
this complex, mainly to stabilize the reaction.
IV. Creatine Bonding Interactions
The
is bound to hBB-CK through a hydrogen bond, and numerous bonds through
water molecules stabilized by side-chains.1
Residues
are responsible for generating binding pocket, which has an innate
specificity for creatine. Another residue, Cys283, attaches
itself to the eta-N nitrogen of creatine. Ser285 pairs with
this Cys283 by binding to its backbone carbonyl and
hydroxyl. Together, these two residues
the creatine and allow for the phosphorylation reaction to occur.
Magnesium (Mg2+) Glu232 also assists in this stabilization,
with its carboxyl forming a hydrogen bond (bidentate
interaction) with the guanidine of creatine.1
This final interaction properly aligns the creatine for
catalysis, allowing the reaction to proceed.
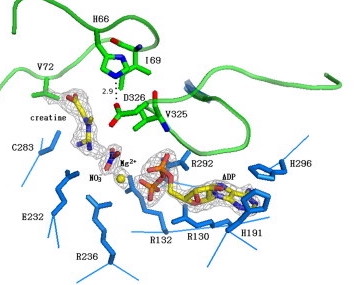
Figure 2: Highlights the
transition state of the creatine-phorylation reaction
interacting with the active site loops of creatine kinase
(green). Magnesium ions (Mg2+) and the nitrate
ions help stabilize the reaction by bridging together the
creatine and ADP substrates.1
V. Clinical Importance
Brain-type creatine kinase is imperative for optimal
brain function. The kinase is responsible for facilitating the
reversible phosphorylation of both ADP and creatine.
Possessing high levels of creatine kinase means that an
organism can effectively manufacture and store phosphorylated
creatine, that can later be converted to ATP for cellular
activity. In the brain, creatine kinase has been found to be
vital for providing ATP for Na+K- ATPase. The ability to
increase and decrease ATP levels within a system allows
creatine kinase to control the ion channels and transports in
brain cells. Research by Aksenov further proves this
stipulation.4 It was found that the brains of
cadavers with both Alzheimer's and Pick's disease had a severe
lack of BB-CK. This was despite there being normal levels of
other variants of creatine kinase in the body. Both these
diseases occur when brain cells lose their ability to
function, and eventually atrophy and die. Other neurological
diseases such as schizophrenia, epilepsy, and even psychosis
have been linked to a deficiency in brain-type creatine
kinase, making it an essential protein for brain health.4
VI. References
1 Aksenov, M. Y., Aksenova,
M. V., Payne, R. M., Smith, C. D., Markesbery, W. R., and
Carney, J. M. (1997). The expression of creatine kinase
isoenzymes in neocortex of patients with neurodegenerative
disorders: Alzheimer's and Pick's disease. Experimental
neurology, 146(2), 458-465.
2 Bong, S. M., Moon, J.
H., Nam, K. H., Lee, K. S., Chi, Y. M., and Hwang, K. Y.
(2008). Structural studies of human brain-type creatine
kinase complexed with the ADP-Mg2+-NO3- -creatine
transition-state analogue complex. FEBS letters,
582(28), 3959-3965.
3 Michael, E., et al.
"Crystal structure of brain-type creatine kinase at 1.41 A
resolution." Protein Science 8.11 (1999): 2258-2269.
4 Hornemann, T.,
Rutishauser, D., and Wallimann, T. "Why is creatine kinase a
dimer? Evidence for cooperativity between the two subunits."
Biochimica et Biophysica Acta (BBA)-Protein Structure and
Molecular Enzymology 1480.1-2 (2000): 365-373. .
5 McLeish, M. J., and
Kenyon, G. L. (2005). Relating structure to mechanism in
creatine kinase. Critical reviews in biochemistry and
molecular biology, 40(1), 1-20.
6 McLaughlin, K., "Creatine
Kinase- the definitive guide"Biology Dictionary
(2022).
Back to Top