TtoA from Thermus thermophilus: A Major Outer Membrane Protein
Kayla Arone '26 and Brooke Heis '26
Contents:
View Type:
I. Introduction
TtoA ,
a major outer membrane protein (OMP) from Thermus thermophilus HB27, is a β-barrel OMP. Integral β-barrel proteins are the most abundant proteins in the outer membrane of modern Gram-negative bacteria. Outer membrane proteins function as channels for nutrients, exporters for proteins, hydrophobic toxic substances, enzymes, or adhesion molecules, and act as assembly factors and anchors to the cell wall. Multiple structures of transmembrane β-barrel outer membrane proteins contain almost all neighboring antiparallel β-strands with a majority of strands within the β-barrel .
The outer membrane of Thermus thermophilus protects the bacteria from the extreme heat environment. The embedded OMP proteins are resistant to denaturing, and help fulfill crucial tasks for the cell (ex. solute and protein translocation). These membrane proteins, like TtoA , fold into antiparallel
that permeate the membrane, instead of transmembrane alpha-helices.
II. General Structure
Thermus thermophilus, TtoA , consists of an eight stranded β-barrel showing an “all-next-neighbor up and down topology” of the strands. One side of the barrel, the strands are connected by three linkages of different lengths. The other side is connected to a large extracellular domain through linkages .
TtoA is orientated in the membrane so that the large-non-barrel domain is orientated to the exterior of the cell, and the β-barrel portion permeates through the . The five-stranded extracellular β-sheet is stabilized by a , and will form crystal contacts that mimic other protein interactions. The extracellular domain also binds to a divalent cation. The top of the extracellular domain has the largest circumference with an elliptic diameter of compared to the β-barrel diameter of .
III. C/N Termini
TtoA contains an N-terminal. This sequence allows for translocation across the inner membrane by the secretion machinery. In addition, TtoA has a signature C-terminus sequence that corresponds to the last β-strand. The
has Phe214 at the C-terminal position, and hydrophobic residues Tyr212, Ala210, Leu208, and Leu206 at respective positions 3, 5, 7 and 9, relative to the C-terminus . The C-terminus is required for binding to the TtOMP85 protein, a requirement for the assembly of TtoA in the membrane. The C-terminus binds to the PORTA domain, and inserts β-strands into the barrel of TtOMP85. The enlarged barrel will then open, and TtoA will dissociate from the protein, and fold into its β-barrel structure within the outer membrane of the cell(Figure 1).
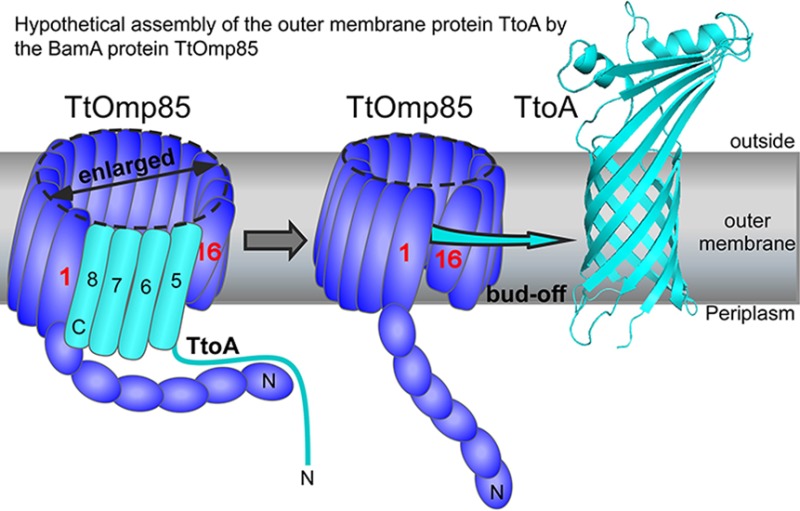
Figure 1. A hypothetical insertion mechanism for the TtOmp85 insertase. In the initial scenario (left panel), the unfolded state of TtoA interacts with the POTRA domains of TtOmp85, while the insertase's barrel remains closed. Moving to the intermediate stage (middle panel), the barrel of TtOmp85 has opened, and the β-sheet has undergone modification. Specifically, the first β-strand of TtoA has been extended by four additional C-terminal β-strands. Simultaneously, the POTRA domains, along with the remaining unfolded N-terminal portion of TtoA, have rotated toward the insertion site. Finally, in the right panel, the process concludes with the dissociation of the folded TtoA from TtOmp85.
IV. Transmembrane Domain
The transmembrane domain of TtoA as a β-barrel structure is composed of with nonpolar side chains facing the lumen pointing towards the membrane and polar side chains facing the interior of the β-barrel. There are two located between the hydrophilic and hydrophobic parts of the membrane lying perpendicular to the barrel axis, showing an orientation normal to the membrane plane. The of the β-barrel with its barrier for hydrophilic compounds and narrow inner surfaces leave no notable space of a continuous channel. The lumen is intersected by residues
pointing from opposite sides toward each other in which a salt bridge is created. Between that intersection, the other space is filled with hydrophobic side chains Val23, Leu82, and Val147. This section of the inner surface of the barrel depicts both steric and electrostatic occlusions and serves as a barrier for hydrophilic compounds.
V. Extracellular Domain
The extracellular domain of TtoA is composed by long strands
of the β-barrel and by the extracellular loops The resulting β-sheet is extended by two additional antiparallel β strands located in extracellular loop 4. There are 92 of the 207 residues present in the extracellular domain. Extracellular loops 2 and 4 contain with a divalent calcium ion. The extracellular domain also contributes two
coming together with the , making up the outer rim of the extracellular domain. Together the extracellular domain of TtoA forms an asymmetric arrangement with an alpha helical side opposed to the extended β-sheet. There is also a divalent cation bound antiparallel to beta strand 9 and 10 in the extracellular loop. These loops form most of the secondary structure elements present. The calcium ions are mostly coordinated in protein structures by aspartate and glutamate residues and altogether show 6 or 7 coordinations. It seems to be bound tightly to TtoA in the outer membrane where the calcium ion is normally present. The asymmetrical orientation of the extracellular domain is also evident in the charge distribution on the surface of TtoA , which exhibits two clusters of negative charges, which include the upper side of the beta sheet and where loops 1, 2 and 4 face each other. The region adjacent to the upper barrel entrance displays a distinctive composition of aromatic and nonpolar side chains
rendering it largely hydrophobic. As it progresses toward the barrel entrance, this area gradually becomes more hydrophilic. The exterior of the barrel is mostly uncharged, and the charged distributions of the downside of the β-sheet and intracellular side is neutral.
VI. References
Koebnik, R et al. “Structure and function of bacterial outer membrane proteins: barrels in a nutshell.” Molecular microbiology vol. 37,2 (2000): 239-53. doi:10.1046/j.1365-2958.2000.01983. Estrada Mallarino, Luisa et al. “TtOmp85, a β-barrel assembly protein, functions by barrel augmentation.” Biochemistry vol. 54,3 (2015): 844-52. doi:10.1021/bi5011305.
Brosig, Alexander, et al. “Crystal structure of a major outer membrane protein from Thermus thermophilus HB27.” Journal of Molecular Biology, vol. 385, no. 5, 6 Feb. 2009, pp. 1445–1455, https://doi.org/10.1016/j.jmb.2008.12.003.
Weber, Irene T., Gary L. Gilliland, James
G. Harman, and Alan Peterkofsky. 1987. Crystal Structure of a
Cyclic AMP-independent Mutant of Catabolite Activator Protein. The
Journal of Biological Chemistry 262:5630-5636.
Back to Top