Following the activation of pro-myostatin, a signaling cascade begins when the mature myostatin growth factor dimer binds with two activin type II receptors (ActIIRA or primarily ActIIRB), phosphorylating the receptors and inducing a conformational change. This allows for the subsequent recruitment and phosphorylation of two activin or TGF-β type I receptors (ALK4 or ALK5 respectively), forming a stable, heterotetrameric signaling complex. The SMAD downstream signaling pathway is activated through the phosphorylation of SMAD 2 and 3 by activated type I receptors. SMAD 2/3 forms a complex with SMAD 4 which is translocated from the extracellular matrix to the nucleus and activates the expression of target genes affecting muscle fiber size. Additionally, SMAD 7 functions as an inhibitory protein, when bound to activated type I and II receptors it will block the activation of SMAD 2/3. 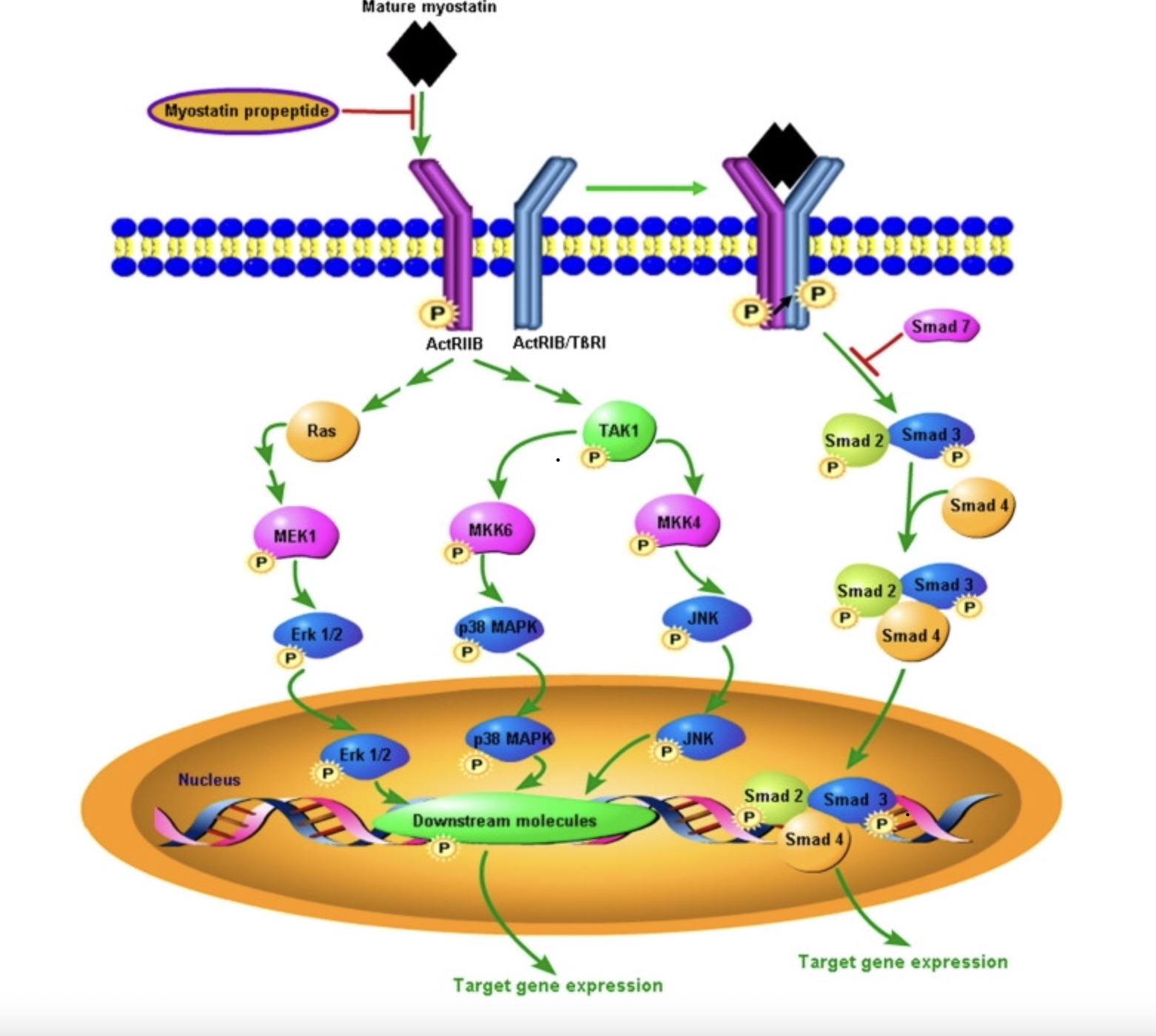
Figure 1: Myostatin signaling pathway (Adapted from Huang et al. 2011).
V. Antagonist Binding
The Myostatin:Follistatin 288 complex made up of two Fst288 molecules wrapped around an active myostatin dimer, blocking the four receptor-binding sites (FSD-1,2,3). FSD1 and FSD2 will contact one myostatin monomer and bury the type II receptor-binding site. Additionally, the N-terminal domain will contact both monomers and bind the type I receptor-binding site. At the type I interface, the fingertip loops of myostatin clamp on the N-terminal domain helix to form three new hydrogen bonds. The side chains of and rearrange to help stabilize the helical conformation causing and to be displayed outwards in different directions. This allows to insert between them which permits the N-terminal domain to interact more closely with myostatin.
VI. Biological Application
The complex regulatory role of myostatin in muscle growth has opened many doors towards medical innovation. Myostatin has been explored in regards to potential therapy for muscle disorders. A common muscle disorder, muscular dystrophy, has been readily studied and scientists have looked at gene therapy as a potential fix. Myostatin antagonist binding can
lead to an increase in muscle mass. Thus scientists have used this knowledge to develop antagonist gene clones delivered through adeno-associated vectors or small, non-pathogenic viruses capable of transferring genetic material into target cells by intramuscular injection. This is believed to have a long-lasting therapeutic effect.
VII. References
Z. Huang, X. Chen, D. Chen, Myostatin: A novel insight into its role in metabolism, signal pathways, and expression regulation. Cellular Signalling. 23, 1441–1446 (2011). Rodgers BD, Ward CW. Myostatin/Activin Receptor Ligands in Muscle and the Development Status of Attenuating Drugs. Endocr Rev. 2022 Mar 9;43(2):329-365. doi: 10.1210/endrev/bnab030. PMID: 34520530; PMCID: PMC8905337.
Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9306-11. doi: 10.1073/pnas.151270098. Epub 2001 Jul 17. PMID: 11459935; PMCID: PMC55416.
Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J. 2009 Sep 2;28(17):2662-76. doi: 10.1038/emboj.2009.205. Epub 2009 Jul 30. PMID: 19644449; PMCID: PMC2738701.
Carnac G, Vernus B, Bonnieu A. Myostatin in the pathophysiology of skeletal muscle. Curr Genomics. 2007 Nov;8(7):415-22. doi: 10.2174/138920207783591672. PMID: 19412331; PMCID: PMC2647158.
Back to Top