[NiFe]-Hydrogenase Huc
Josiah Cox '27 & Matteo '26
Contents:
I. Introduction
Hydrogenases represent a family of metalloenzymes which catalyze the reversible oxidation of molecular hydrogen (H2 ↔ 2e− + 2H+) using Fe or Fe and Ni atoms in their active sites (Cracknell et al., 2009). Nine identified phyla of aerobic bacteria including Proteobacteria and Cyanobacteria express these enzymes. Bacteria use these enzymes to derive low potential electrons from atmospheric H2 which they use to drive electrochemical gradients in oxidative phosphorylation, power carbon fixation, and obtain redox balance during photosynthesis (Greening et al., 2023; Grinter et al., 2023). This oxidative process accounts for 75% of the annual removal of H2 from the atmosphere or roughly 60 Tg (Grinter et al., 2023). Such a process provides bacteria in nutrient poor environments including hyper-arid polar soils with supplemental energy necessary for mixotrophic growth or retaining viability during periods of dormancy spurred by starvation (Grinter et al., 2023).
One such bacteria includes the ubiquitous soil-dwelling Mycobacterium smegmatis (M. smegmatis). This bacteria utilizes the hydrogenase, hydrogen menaquinone oxidoreductase (Huc), to sequester electrons from H2 and donate them to oxidative phosphorylation for ATP synthesis (Figure 1; Grinter et al., 2023). Huc is expressed in M. smegmatis during periods of cell growth and becomes transcriptionally upregulated as heterotrophic energy sources become less abundant in their diet (Cordero et al., 2019). Huc uses Fe and Ni atoms for oxidative catalysis, making the enzyme an [NiFe]-hydrogenase (Grinter et al., 2023). Importantly, oxygen acts as a catalytic poison for [NiFe]-hydrogeases by over-oxidizing these [NiFe] metal centers. This results in the subsequent attachment of reducing agents to these centers, inhibiting oxidative catalysis. Yet, Huc remains active in aerobic conditions (Cracknell et al., 2009; Wulff et al., 2014).
This is possible because Huc’s metal centers are embedded deep within its structure. The centers are connected to the external world through miniscule hydrophobic gas tunnels which block oxygen’s access but permit hydrogen entrance (Grinter et al., 2023). Post oxidation, these metal centers relay electrons down a series of [3Fe-4S] clusters into a hydrophobic chamber contained within Huc, associated with the phospholipid membrane of M. smegmatis. Importantly, the amphipathic menaquinone, an integral cofactor of M. smegmatis oxidative phosphorylation is commonly dissolved in the lipid bilayer core. Because of this, menaquinone may diffuse into this hydrophobic chamber. Once inside, it is reduced by these clusters to menaquinol before diffusing back into the membrane core (Figure 2A). Here, menaquinol is oxidized by the cytochrome bcc-aa3 oxidase to generate a proton gradient and eventually ATP (Figure 1; Grinter et al., 2023).
Many fuel cells yield energy through the splitting of molecular hydrogen using expensive platinum-based oxygen sensitive catalysts. For this reason, Greening et al. (2023) have proposed that Huc may act as a cheaper and more efficient replacement for platinum in fuel cell catalytics.
All of the following structure / function information surrounding [NiFe]-Hydrogenase Huc has been sourced from cryo-electron microscopy work by Grinter et al. (2023). To effectively view a given section, please press the “reset protein” button at the top of each one before reading on.
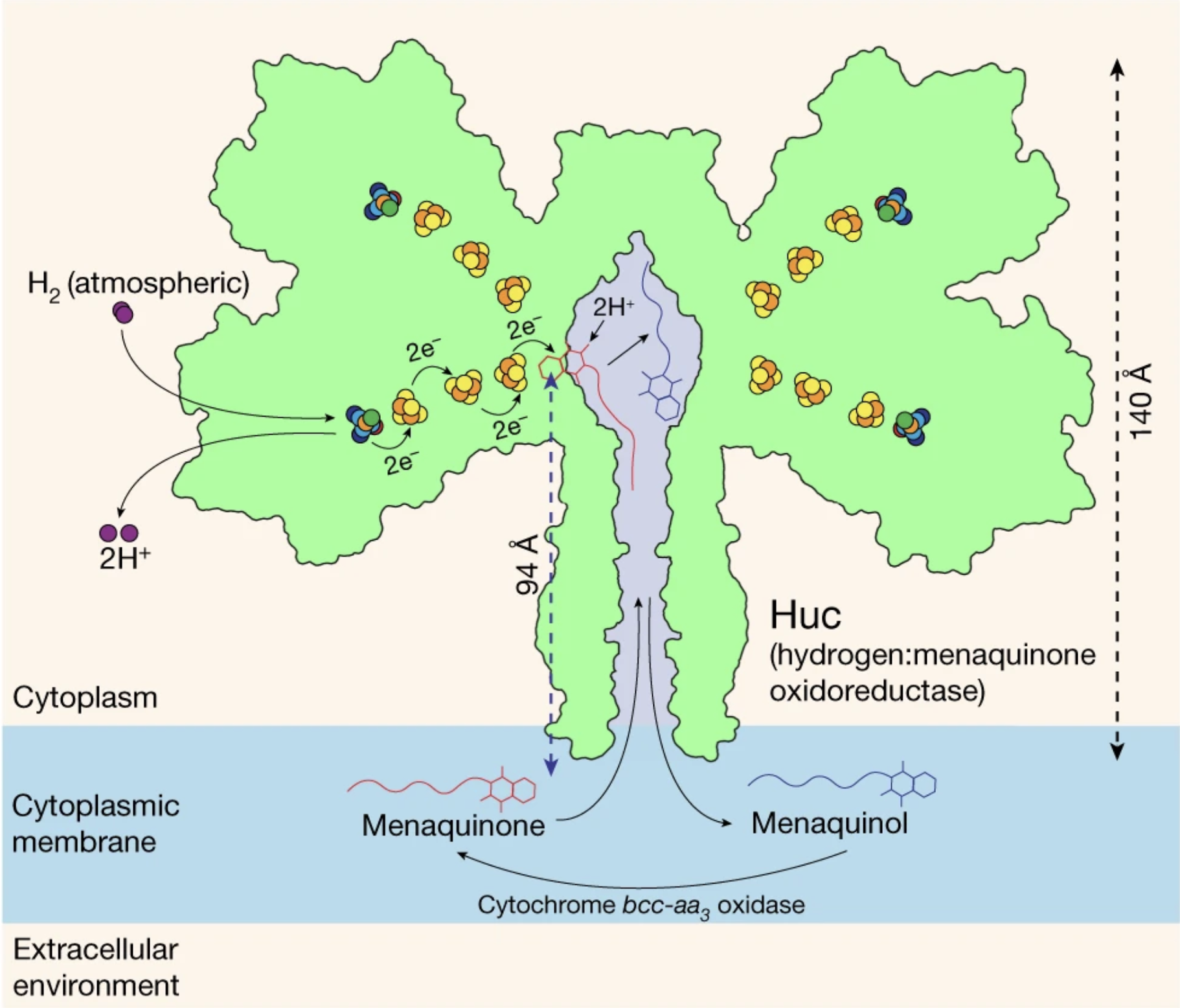 Figure 1: [NiFe]-Hydrogenase Huc participating in H2 oxidation, electron relay, and menaquinone reduction. Image produced by Grinter et al. (2023).
Figure 1: [NiFe]-Hydrogenase Huc participating in H2 oxidation, electron relay, and menaquinone reduction. Image produced by Grinter et al. (2023).
II. General Structure
[NiFe]-Hydrogenase Huc is a membrane associated cytoplasm facing 833 kDa hetero 20-mer that is 7,360 residues in length. 3 unique subunit types are contained within Huc: (58 kDa), (39 kDa), and (18 kDa). Each HucL subunit contains 1 metal center and is responsible for H2 oxidation. Each HucS subunit contains 3 clusters and is responsible for electron relay and menaquinone reduction. Finally, each HucM subunit is membrane bound. Together the HucMs form a hydrophobic chamber responsible for menaquinone entrance and accessibility to the distal reductive [3Fe-4S] cluster of HucS. Huc may be divided into 4 α-helix rich and a . Each lobe contains 2 structures referred to as Huc promoters, and each Huc promoter contains 1 HucS and 1 HucL subunit for a total of 8 HucLs and 8 HucSs per hydrogenase. These HucLS heterotetramer lobes are bound to the amino termini regions of 4 HucMs which comprise the central stalk through hydrophobic interaction. These α-helical hydrophobic residue rich subunits intertwine to form a cage-like structure who’s carboxyl termini are partially inserted into the membrane of M. smegmatis.
III. Hydrogen Oxidation
The metallic which facilitates H2 oxidation at the center of a HucL subunit is composed of an Fe and an Ni atom. A center’s Fe atom is centrally coordinated by partial covalent-ionic iron-sulfur bonds with spanning residues 65-61 and 496-515 within its containing HucL subunit. The stability of these α-helices is maintained by a spanning residues 432-452. The specific residues from the α-helices involved in metal center binding include thiolate ligands. Similarly, a center’s Ni atom remains coordinated by nickel-sulfur bonds from Cys65 and Cys513, as well as . If oxygen were to arrive at the center under electron rich-conditions, it may oxidize the center resulting in the reductive ligand, HO-, becoming bound in a bridging position between the Fe and Ni atoms. Such bridging inhibits the oxidizing potential of the center, but can be rapidly recovered from H2 exposure. This version of the center is referred to as the Ready state. Under electron-deficient contitions in which oxygen arrives at the center, it may catalyze the attachment of a bridging peroxo group; this version of a center is in the Unready state. Metal centers in the Unready state are also oxidatively inhibited, but are orders of magnitude slower to recovery (Cracknell et al., 2009; Wulff et al., 2014). These states are avoided in [NiFe] Huc Hydrogenase because its metal centers are deeply embedded within the 58 kDa HucL subunit of each Huc promoter. The centers are connected to the external world through 27Å long gas tunnels rich in hydrophobic residues. These residues partially deter the entrance of aqueous electronegative O2 but allow entrance of less electronegative molecules such as H2. These tunnels converge 5Å preceding the metal center. At this , residues Glu15 and Ile64 form a bottleneck 2Å in diameter. This passage is far too small for O2 at 3.5Å in diameter, but just large enough for the 2Å H2 to squeeze by.
IV. Electron Relay
Once 2 electrons are removed from H2 in HucL, they are relayed across a series of 3 clusters in HucS. The proximal cluster, is coordinated by covalent iron-sulfur bonds between its 3 Fe atoms, and the S atoms of residues . Additionally, in the HucL subunit an isomer of histidine, stabilizes the proximal cluster through a hydrogen bond between its amino group and an S atom of the cluster. It has also been found in some hydrogenases that this particular D-His165’s carboxyl terminus hydrogen bonds with a ligand near the medial cluster. This ligand is unknown, but it is thought to have a role in tuning the cluster’s redox potential towards slow but high electron affinity kinetics. This proximal cluster acts as the first electron acceptor from [NiFe]. It donates these electrons across a 10Å space to the medial cluster coordinated by . The distal cluster, 8Å away receives these electrons from the medial cluster, and itself is coordinated by .
V. Stalk Properties
The HucLS heterotetramer lobes containing the [NiFe] centers and [3Fe-4S] clusters are bound to the amino termini regions of which themselves form a homotetramer. These HucM subunits intertwine to form a cage-like structure which comprises the central stalk of Huc and anchors it to the phospholipid bilayer of M. smegmatis. Each HucM contains α-helices near their carboxyl termini rich in charged residues including . These residues face outwards from the cage and anchor Huc to the bilayer by participating in hydrogen bonds with the polar heads of the layer’s lipids. The further strengthens this attachment by participating in hydrophobic interactions with the membrane’s hydrophobic core.
VI. Menaquinone Reduction
The is also rich in toward the cage’s center forming the walls of a hydrophobic chamber. These residues include isoleucine and leucine. Because the integral oxidative phosphorylation cofactor contains a highly hydrophobic backbone and is dissolved within the lipid bilayer core, it is free to diffuse from the membrane into this chamber. Once diffused, menaquinone associates with the menaquinone of HucS which is inserted directly into the HucM homotetramer hydrophobic chamber and contains the distal [3Fe-4S] cluster. In this pocket, the headgroup of menaquinone receives . Specifically, menaquinone forms a hydrogen bond between one of its carbonyl groups and the terminal hydroxyl of Tyr347. The other carbonyl group forms a stabilizing carbonyl-carbonyl n→π* interaction with the backbone carbonyl of Lys258. The indole ring of menaquinone’s head group also forms imperative stabilizing π–π interactions with the aromatic sidechain of the residue. This positions menaquinone’s headgroup 6Å from the [3Fe-4S] cluster, permitting the reduction of its carbonyl groups to hydroxyls, forming menaquinol (Figure 2A). Interestingly, in the absence of menaquinone, Tyr275 adopts a second conformation which is thought to block the distal cluster from reducing any O2 which might have diffused into the binding pocket (Figure 2B & C). 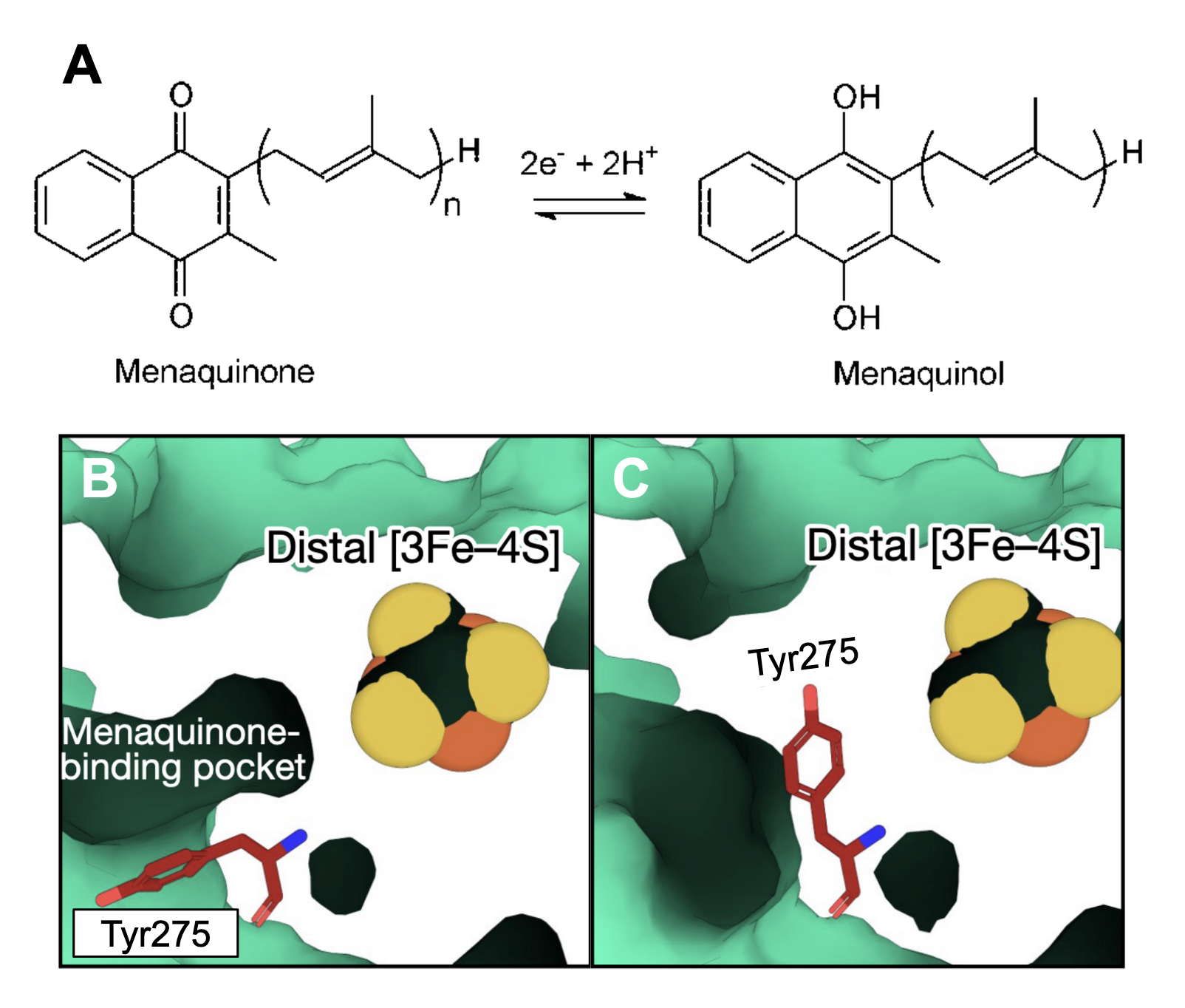 Figure 2: (A) Menaquinone reduction to Menaquinol; image produced by Joshi et al. (2018). Menaquinone binding pocket with Tyr275 in (B) the stabilizing π–π conformation or (C) the O2 blocking conformation.
Figure 2: (A) Menaquinone reduction to Menaquinol; image produced by Joshi et al. (2018). Menaquinone binding pocket with Tyr275 in (B) the stabilizing π–π conformation or (C) the O2 blocking conformation.
VII. References
Cordero PRF, Grinter R, Hards K, Cryle MJ, Warr CG, Cook GM, Greening C. Two uptake hydrogenases differentially interact with the aerobic respiratory chain during mycobacterial growth and persistence. Journal of Biological Chemistry. 2019;294(50):18980–18991. doi:10.1074/jbc.RA119.011076 Cracknell JA, Wait AF, Lenz O, Friedrich B, Armstrong FA. A kinetic and thermodynamic understanding of O2 tolerance in [NiFe]-hydrogenases. Proceedings of the National Academy of Sciences. 2009;106(49):20681–20686. doi:10.1073/pnas.0905959106
Greening C, Kropp A, Vincent K. Developing high-affinity, oxygen-insensitive [NiFe]-hydrogenases as biocatalysts for energy conversion. Biochemical Society Transactions. 2023;51(5):1921–1933. doi:10.1042/BST20230120
Grinter R, Kropp A, Venugopal H, Senger M, Badley J, Cabotaje PR, Jia R, Duan Z, Huang P, Stripp ST, et al. Structural basis for bacterial energy extraction from atmospheric hydrogen. Nature. 2023;615(7952):541–547. doi:10.1038/s41586-023-05781-7
Joshi S, Fedoseyenko D, Mahanta N, Manion H, Naseem S, Dairi T, Begley TP. Novel enzymology in futalosine-dependent menaquinone biosynthesis. Current Opinion in Chemical Biology. 2018;47:134–141. doi:10.1016/j.cbpa.2018.09.015
Kruse S, Goris T, Wolf M, Wei X, Diekert G. The NiFe Hydrogenases of the Tetrachloroethene-Respiring Epsilonproteobacterium Sulfurospirillum multivorans: Biochemical Studies and Transcription Analysis. Frontiers in Microbiology. 2017 [accessed 2024 Dec 14];8. http://journal.frontiersin.org/article/10.3389/fmicb.2017.00444/full. doi:10.3389/fmicb.2017.00444
Ogata H, Lubitz W, Higuchi Y. Structure and function of [NiFe] hydrogenases. Journal of Biochemistry. 2016;160(5):251–258. doi:10.1093/jb/mvw048
Truglio JJ, Theis K, Feng Y, Gajda R, Machutta C, Tonge PJ, Kisker C. Crystal Structure of Mycobacterium tuberculosis MenB, a Key Enzyme in Vitamin K2 Biosynthesis. Journal of Biological Chemistry. 2003;278(43):42352–42360. doi:10.1074/jbc.M307399200
Wulff P, Day CC, Sargent F, Armstrong FA. How oxygen reacts with oxygen-tolerant respiratory [NiFe]-hydrogenases. Proceedings of the National Academy of Sciences. 2014;111(18):6606–6611. doi:10.1073/pnas.1322393111
Back to Top