Insulin Receptor Kinase
Lucy Cassell-Kelley, Amelia Stevenson
View Type:
Contents:
I. Introduction
II. General Structure
III. Activation State
IV. Kinase Activity
V. ATP Binding
VI. References
I. Introduction
The insulin tyrosine receptor kinase acts as the first step in the receptor tyrosine kinase
(RTK) pathway, which helps to regulate cell growth and metabolism. The insulin receptor is
activated through the allosteric binding of insulin. The insulin binding induces a
conformational change of the receptor and stimulates the enzyme’s kinase activity.
The kinase activity of the receptor then phosphorylates serine and threonine residues on
insulin receptor substrate (IRS) proteins. Once phosphorylated, IRS proteins can bind to
signaling partners and amplify/reduce the insulin signal, allowing for insulin-binding-induced
regulation of glucose uptake and cellular glycolysis.
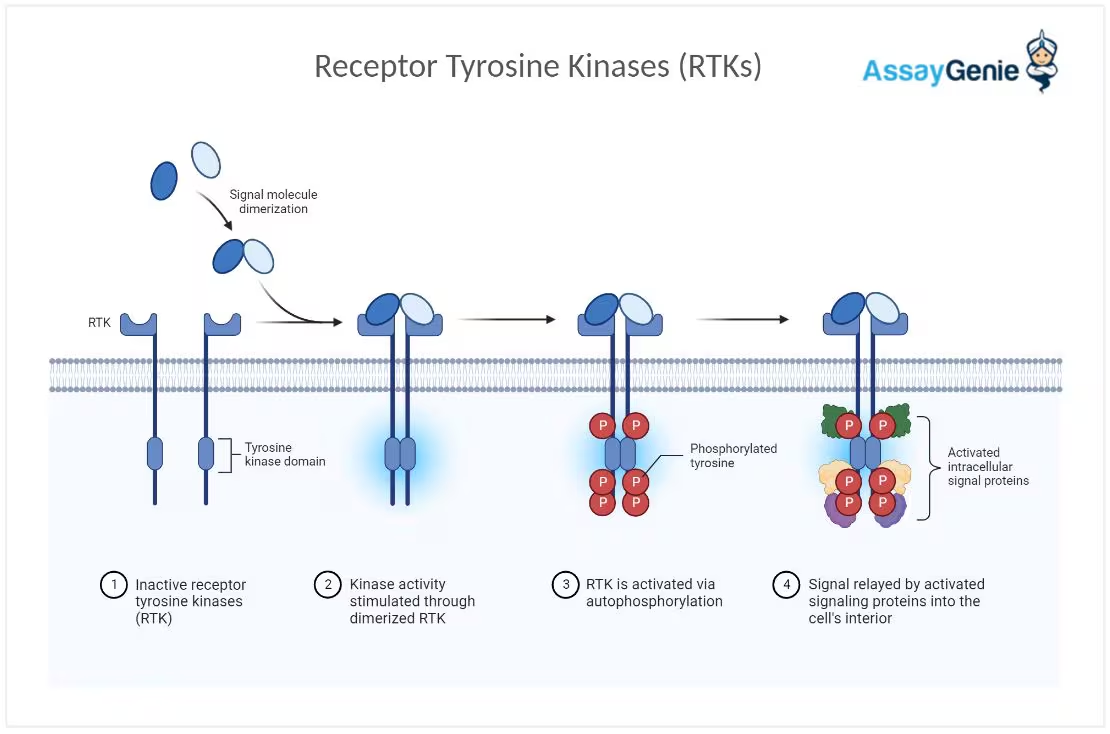
Fig. 1. Receptor Tyrosine Kinase Pathway.
II. General Structure
The insulin receptor kinase functions as a dimer, with an N-terminal lobe comprised of the
alpha subunits and a C-terminal lobe comprised of the beta subunits. The alpha and beta
subunits are connected through covalent disulfide bonds, with several disulfide bonds
binding the two alpha subunits and one disulfide bond stabilizing the alpha and beta subunits.
The N-lobe
and alpha subunits contain the ligand binding domain, while the C-lobe
and beta
subunits contain the catalytic or kinase domain. The N-lobe forms a
five-stranded anti-parallel B-sheet
with one alpha-helix.
The N-lobe alpha helix helps regulate ligand binding at the active site. Inactivation
of binding occurs through the movement of the alpha helix outwards from the active site,
causing disruptions in interactions between the lobes and a reduction in the kinase activity.
The C-terminal lobe is primarily alpha-helical, containing seven alpha-helices and
four small beta sheets , which house the catalytic amino acid residues essential for kinase activity and phosphorylation.
III. Activation State
Insulin allosterically binds to the C-terminal alpha subunit located in the N-lobe.
The insulin binding causes the receptor to undergo a conformational change, activating its B-subunit, which houses the catalytic/kinase domain. The kinase domain is activated through the autophosphorylation of three tyrosine residues within the kinase domain's activation loop (A-loop), which is found between the N and C lobes, driven by the ligand-induced conformational change. The autophosphorylation of
in the A-loop stimulates the receptor's kinase activity. Once insulin binds and causes the autophosphorylation of Tyr1158, 1162,
and 1163, the A-loop is removed from the catalytic site, allowing ATP and substrates to
bind to their active sites, increasing kinase activity. Phosphoryalted Tyr1163 stabilizes
the tris-phosphorylated A-loop through
to , a side chain on one
side of the loop and another bond to the backbone amide nitrogen of
.
Tyr1162 also exhibits hydrogen bonding, with its phosphate group making two hydrogen bond contacts to .
IV. Kinase Activity
There is a family of receptor tyrosine kinases that have the Tyrosine kinase domain. The insulin receptor falls into this family of receptor tyrosine kinase, where the Beta subunits contain the tyrosine kinase domains. These domains are responsible for the autophosphorylation that occurs. But before that, when insulin binds to the alpha subunits of the receptor, a conformational change occurs, which leads to the activation of the intrinsic kinase activity of the beta subunits. Receptor tyrosine kinases are allosterically regulated by their cognate ligands and function as dimers. The initial response to the ligand is receptor autophosphorylation for all receptor tyrosine kinases. This tyrosine kinase domain activates and phosphorylates target proteins, initiating the cellular insulin response. The target proteins bind to a pocket in the C-lobe, primarily interacting with the residues
V. ATP Binding
When the receptor is inactive and unbound from insulin, the A-loop remains unphosphorylated and is inserted into the catalytic site of the kinase, blocking the substrate and ATP binding sites, which are necessary for kinase activity. The ATP binds to a hydrophobic pocket within the beta subunit of the protein, directly interacting with . In the presence of insulin, the receptor undergoes a conformational change, allowing access to the ATP binding domain located in the beta subunit of the receptor. The receptor’s beta subunit takes a phosphate group from the ATP, using it as a phosphate donor for the autophosphorylation of tyrosine, thus activating the kinase domain. ATP is a necessary substrate for the receptor’s kinase activity.
VI. References
Hubbard SR, Wei L, Ellis L, Hendrickson WA. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994 Dec 22-29;372(6508):746-54. doi: 10.1038/372746a0. PMID: 7997262.
“Insulin Receptor Signaling.” Cell Signaling Technology, www.cellsignal.com/pathways/insulin-receptor-signaling-pathway#:~:text=Pathway%20Description%3A&text=Insulin%20activates%20the%20insulin%20receptor,sites%20for%20numerous%20signaling%20partners. Accessed 9 Dec. 2024.
Kornev, Alexandr P, and Susan S Taylor. “Defining the Conserved Internal Architecture of a Protein Kinase.” Biochimica et Biophysica Acta, U.S. National Library of Medicine, Mar. 2010, pmc.ncbi.nlm.nih.gov/articles/PMC3435107/#:~:text=The%20two%20lobes%20form%20a,exchange%20during%20the%20catalytic%20cycle.
Meyts, Pierre De. “The Insulin Receptor and Its Signal Transduction Network.” Endotext [Internet]., U.S. National Library of Medicine, 27 Apr. 2016, www.ncbi.nlm.nih.gov/books/NBK378978/.
PF;, Lee J;Pilch. “The Insulin Receptor: Structure, Function, and Signaling.” The American Journal of Physiology, U.S. National Library of Medicine, pubmed.ncbi.nlm.nih.gov/8141246/#:~:text=Abstract,differentiation%2C%20growth%2C%20and%20metabolism. Accessed 9 Dec. 2024.
Riaz, Shanza, and Sean Mac Fhearraigh PhD. “Insulin Signaling and RTK: An Overview.” Assay Genie, www.assaygenie.com/blog/insulin-signaling-and-rtk#:~:text=The%20insulin%20receptor%20is%20a%20tyrosine%20kinase%20receptor%20(RTK)%20that,protein%20tyrosine%20phosphorylation%20and%20glycolysis. Accessed 9 Dec. 2024.
Back to Top