Streptomyces castaneoglobisporus Tyrosinase and ORF378 Caddie Protein
M Vu '27 and Erin Frank '27
Contents:
I. Introduction
Tyrosinase (also called monophenol monooxygenases) are type 3 copper proteins with
two copper ions in the active site. They catalyze the conversion of monophenols (e.g., tyrosine) into o-diphenols (monophenolase activity),followed by the oxidation of the o-diphenols to the corresponding o-quinone derivatives. Starting from tyrosine, the final product of the tyrosinase-catalyzed reaction is dopaquinone, which is a precursor of melanin.
Tyrosinase is located in melanocytes, which are specialized cells that produce melanin. In mammals, melanin is found in the skin, hair follicles, the colored part of the eye (the iris), and the retina. With this knowledge, melanin has been a topic of interest for the field of medicine and skin cosmetics, specifically for its role in skin pigmentation abnormalities, such as vitiligo, hyperpigmentation, and oculocutaneous albinism.
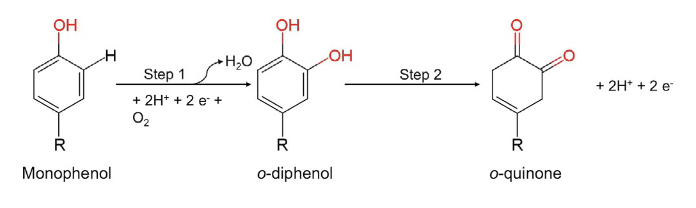
Fig.1. Reaction catalyzed by tyrosinase.
II. General Structure
The overall structure of
can be divided into three domains:
,
, and
. The four-helix bundle central domain, which is composed of six conserved
, acts as a binding site for the copper oxidizing ions . This dicopper-binding center is positioned at the bottom of the large
as a putative substrate-binding pocket formed by the hydrophobic residues, which also serves to stabilize the N-terminal and C-terminal B-sheet structure.
, a caddie protein that covers the molecular surface of tyrosinase, seems to competitively inhibit substrate binding to the active site of the protein. ORF378 is composed of one six stranded β-sheet as well as one α-helix α-helix (
). The
between tyrosinase and ORF378 is mediated by Tyr98 on ORF378 which protrudes from the core region of ORF378 and stacks with the imidazole ring of His194 from tyrosinase . When the tyrosinase - ORF378 complex forms, the decrease in surface area is only 980 Å2, which is lower than the typical surface area decrease in dimers, suggesting that the interaction between tyrosinase and ORF378 is relatively weak.
III. Catalytic Activity
The active center, or dinuclear copper center, facilitates the
of each
copper ion by the imidazole nitrogens of
histidine residues
. The active site exists in the three forms depicted below(deoxy, oxy and met) as the reaction proceeds.
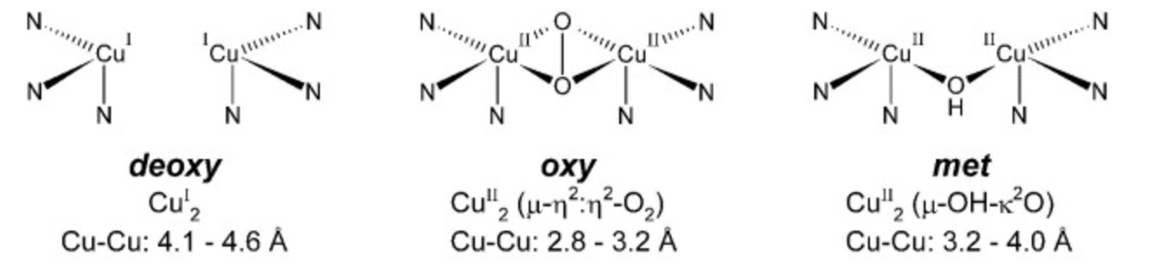
Figure 2: Deoxy, Oxy and Met forms of Tyrosinase Active Center
The reaction procedes as follows...
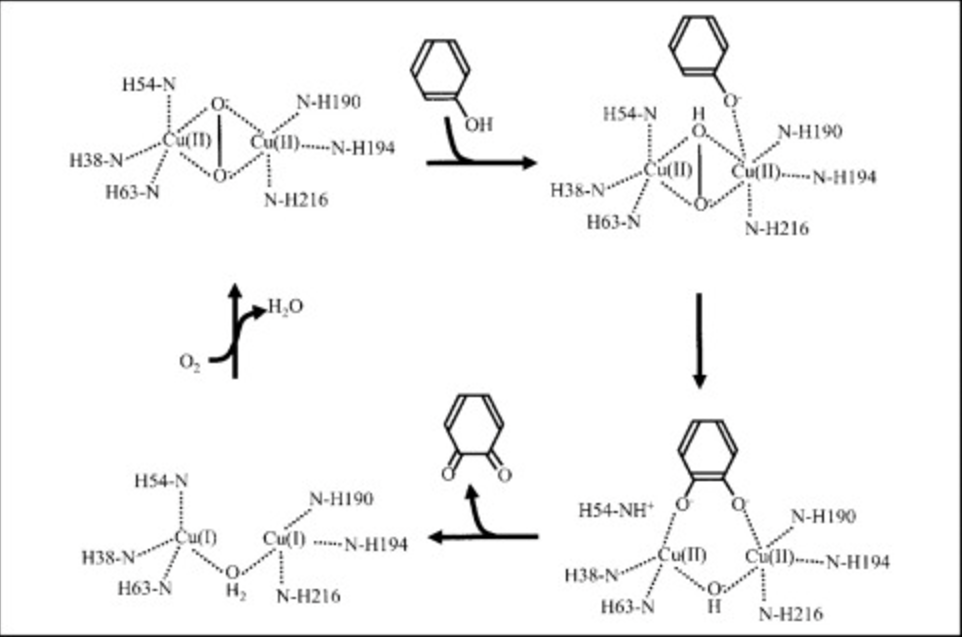
Figure 3: Monophenolase and monoxgyenase reaction in Tyrosinase Active Center
The tyrosinase model here exists in met form, which is assumed to be its resting form. The two
Cu ions are
by two
water molecules that are being held in place through
with Tyr98 of ORF378.
It is crucial that the bonds holding the
Cu ions in place are flexible for the functional enzymatic activity of tyrosinase, as the active site must accommodate space for two
oxygen atoms during both monophenolase and monooxygenase activities.
IV. ORF378 As A Copper Transporter
In addition to the two
Cu(II) atoms in the catalytic center of tyrosinase, there exists two more Cu(II) binding sites in the Tyrosinase-ORF378 complex.
The first of these two binding sites exist on the surface of
ORF378.
Cu(II)
to the nitrogen atom from
His82 of
ORF378.
The nitrogen atom of
His82 side chain forms a
with the nitrogen atom of
His97 of
ORF378 by mediation of a nitrate ion.
The second site, which is
empty in this model, exists at
Met84 of ORF378 where
Cu(II) binds to a
.
In order for this interaction to occur, the
must be rotated around the bond between their Cβ and Cγ atoms.
The movement of additional
Cu(II) atoms from the first to the second binding site is time-dependent, suggesting that the Cu(II) binding sites on
ORF378 may exist to transfer
Cu(II) to the catalytic center of
Tyrosinase.
Additionally, in the copper-free Tyrosinase-ORF378 complex,
Ile42 and
His54 of
Tyrosinase and
Val94 of
ORF378 have
.
This is especially important in the alternate side chain of
His54 of
Tyrosinase because it comes into
with the side chain of
His97 from
ORF378.
These clustered
His residues, along with
His66 and
His68 which extend from the molecular surface of
ORF378 to the active center of
Tyrosinase Cu(II) ions from
ORF378 to the catalytic site of
Tyrosinase.
V. Albinism-Causing Mutations in Tyrosinase
Oculocutaneous albinism type I (OCA1) is an incurable autosomal recessive disorder that disrupts the normal production of melanin, which reduces coloring of the hair, skin, and eyes and causes problems with vision. More than 100 mutations in the TYR gene, including missense, nonsense, frameshift and splice site mutations, have been identified in people with OCA1. Gene therapy is anticipated to be a promising treatment for this disorder in future years. The use of gene correction techniques such as retrovirus vectors, adenovirus vectors and CRISPR/Cas9 system can edit mutations (erroneous segments) in the genes encoding tyrosinase to increase melanin production.
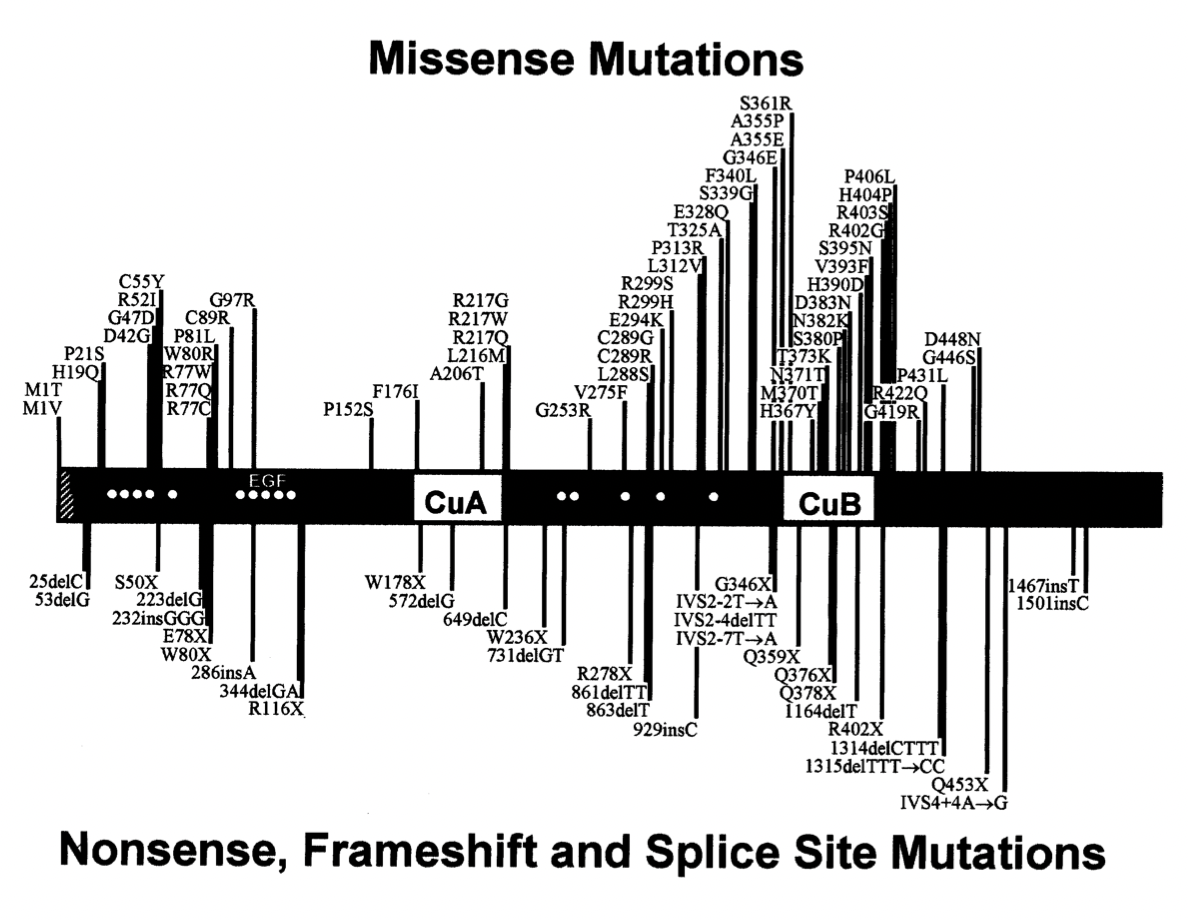
Fig.4. Location of the mutations of the TYR gene associated with OCA1. Missense mutations are on the top and nonsense, frameshift and splice site mutations are on the bottom.
VI. References
Ismaya, W. T., RozeboomH. J., Weijn, A., Mes, J. J., Fusetti, F., Wichers, H. J., & Dijkstra, B. W. (2011). Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry, 50(24), 5477–5486. https://doi.org/10.1021/bi200395t Lai, X., Wichers, H. J., Soler-Lopez, M., & Dijkstra, B. W. (2017). Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chemistry - a European Journal, 24(1), 47–55. https://doi.org/10.1002/chem.201704410.
Matoba, Y., Kihara, S., Bando, N., Yoshitsu, H., Sakaguchi, M., Kayama, K., Yanagisawa, S., Ogura, T., & Sugiyama, M. (2018). Catalytic mechanism of the tyrosinase reaction toward the Tyr98 residue in the caddie protein. PLOS Biology, 16(12), e3000077. https://doi.org/10.1371/journal.pbio.3000077
Kanteev, M., Goldfeder, M., & Fishman, A. (2015). Structure-function correlations in tyrosinases. Protein Science, 24(9), 1360–1369. https://doi.org/10.1002/pro.2734
Matoba, Y., Kumagai, T., Yamamoto, A., Yoshitsu, H., & Sugiyama, M. (2006). Crystallographic Evidence That the Dinuclear Copper Center of Tyrosinase Is Flexible during Catalysis. Journal of Biological Chemistry, 281(13), 8981–8990. https://doi.org/10.1074/jbc.m509785200
Pretzler, M., & Rompel, A. (2018). What causes the different functionality in type-III-copper enzymes? A state of the art perspective. Inorganica Chimica Acta, 481, 25–31. https://doi.org/10.1016/j.ica.2017.04.041
Back to Top