Human Menin 3U84
Olivia Ide '27 and Raine Hammel '25
Contents:
I. Introduction
Menin, a multifunctional protein encoded by the MEN1 gene, plays diverse roles in cellular and molecular processes, including tumor suppression, gene regulation, epigenetics, and cellular signaling. Menin is expressed in various organs, with its expression and function differing widely. Notably, its unique domain structure, distinct from other proteins, has made understanding its biological function challenging. As a tumor suppressor, Menin's inactivation is linked to multiple endocrine neoplasia type 1 (MEN1), an autosomal dominant tumor syndrome characterized by endocrine gland tumors. Menin exerts its tumor-suppressive effects partly through interactions with other proteins, such as the transcription factor JUND, which shifts from a growth promoter to a tumor suppressor when bound to Menin. Additionally, Menin interacts with mixed lineage leukemia protein 1 (MLL1), a histone H3 lysine 4 methyltransferase, serving as an oncogenic cofactor in MLL1-fusion-protein-induced leukemogenesis. It also facilitates MLL1 binding to chromatin through the lens epithelium-derived growth factor (LEDGF), highlighting Menin's role as a coordinator of complex protein interactions. This multifunctionality, combined with its involvement in tumor suppression and oncogenesis, has made Menin an attractive target for drug development aimed at treating a range of cancers.
II. General Structure
Menin is an average sized protein that contains 610 amino acid residues. Menin is made up of both α helices
and 𝛽 strands.
Menin is highly conserved across species, as it is expressed in all tissues, but its levels differ across various tissues.
Menin consists of , N-terminal domain, a transglutminase-like domain, a TPR motif domain, and a C-terminal domain. The N-terminal domain has a
and interacts with many other proteins like MLL1 and LEDGF.
The TPR motifs
form the core of the protein and are composed of of anti-parallel α helices. Menin also contains it's
which are located near it's C-terminal.
These NLSs act like a 'tag' on the exposed surface of a protein to target the protein to the cell nucleus through the nuclear pore complex and to direct it into the nucleus via its recognition by cytosolic nuclear transport receptors and can interact with DNA.
III. Tumor Suppression
Menin is associated with the prevention of tumor formation, particularly in endocrine tissues. Mutations of Menin are associated with Men1 syndrome, which is a tumor predisposition syndrome characterized by hyperplasia and growth of tumors on the pituitary gland. Menin regulates gene transcription in a variety of ways. It can bind specific DNA binding proteins along with histone methyltransferases to target the loci of p18ink4c, p27kip1and Hoxc8 genes in chromatin. Activation of these genes leads to cell growth inhibition. Menin can also bind with a histone deacetylase with a DNA binding protein to target the repression of hTERT and IGFBP-2 genes. The consequences of Menin's tumor supression can lead to reduced cell proliferation, genomic instability, and apoptosis
IV. Protein interactions
Menin performs its biological role by binding to various protein partners, with recent studies demonstrating that its
likely serves as the binding site for some of these partners. The cavity is formed by multiple α-helices
from the TRP motifs
and the N- and C-terminal α-helical
bundle domains. The character of this site is defined by hydrophobic residues (Leu-177, Pro-245, Leu-257, Leu-286, Tyr-276, Met-278, Tyr-319, Met-322, Tyr-323, and Phe-328) and acidic side chains (Asp-136, Asp-153, Asp-180, Asp-285, Glu-288, Glu-359, Glu-363, and Glu-366). The combination of the hydrophobic residues and acidic side chains contributes to the site's strong negative surface potential. The interaction between Menin and MLL (Mixed Lineage Leukemia protein) plays a vital role in gene regulation, particularly in the expression of HOX genes, which are critical for development and cell differentiation. MLL, a transcriptional regulator, catalyzes the methylation of histone H3 lysine 4 (H3K4), an epigenetic mark associated with active gene transcription. In leukemias involving MLL rearrangements (MLL-X), MLL fuses with partner genes to form oncogenic fusion proteins that retain the ability to interact with Menin. This interaction leads to the misregulation of genes, including HOXA7, HOXA9, HOXA10, HOXC7 and MEIS1,
driving uncontrolled cell proliferation and leukemia (Figure 1).
Studies have shown that Menin recognizes and binds to a Menin-binging motif (MBM1) located on the N-terminal.
More specifically, mutations of Ser155, Met278, and Tyr323
indicate that these regions are crucial for MBM1 binding. 
Figure 1. The MLL-protein complex recruits and binds to chromatin modifying complexes and histone methyltransferases that result in a promoted RNA polymerase II, which allows for productive elongation and activation of MLL-fusion target genes that cause differentiation block such as HOX, MEIS1, and BCL2, allowing for cells to proliferate.
V. Cancer Research
Menin is a critical target in cancer research because of its dual role as a tumor suppressor and a facilitator of oncogenesis in specific contexts. Mutations in the MEN1 gene, which encodes Menin, lead to multiple endocrine neoplasia type 1 (MEN1), a hereditary cancer syndrome characterized by tumors in endocrine tissues. As mentioned previously, Menin interacts with the MLL protein, regulating genes involved in cell growth and differentiation. In leukemia, MLL fusion proteins exploit this interaction to aberrantly activate oncogenes, driving cancer progression. Targeting Menin, particularly its interaction with MLL fusion proteins, offers a promising therapeutic approach. This significance positions Menin as a key focus in developing treatments for MLL-rearranged leukemias. Menin inhibitors are new targets in Acute Myeloid Leukemia (AML) therapies to date. AML is characterized by abnormal myeloid differentiation patterns. AML accounts for 1% of cancers, with ~11,000 deaths per year. Revumenib, an early-phase clinical trial drug, stops the oncogenes affected by the mutated protein from being expressed initially by binding to Menin and permitting it to bind to the MLL complex (Figure 2). 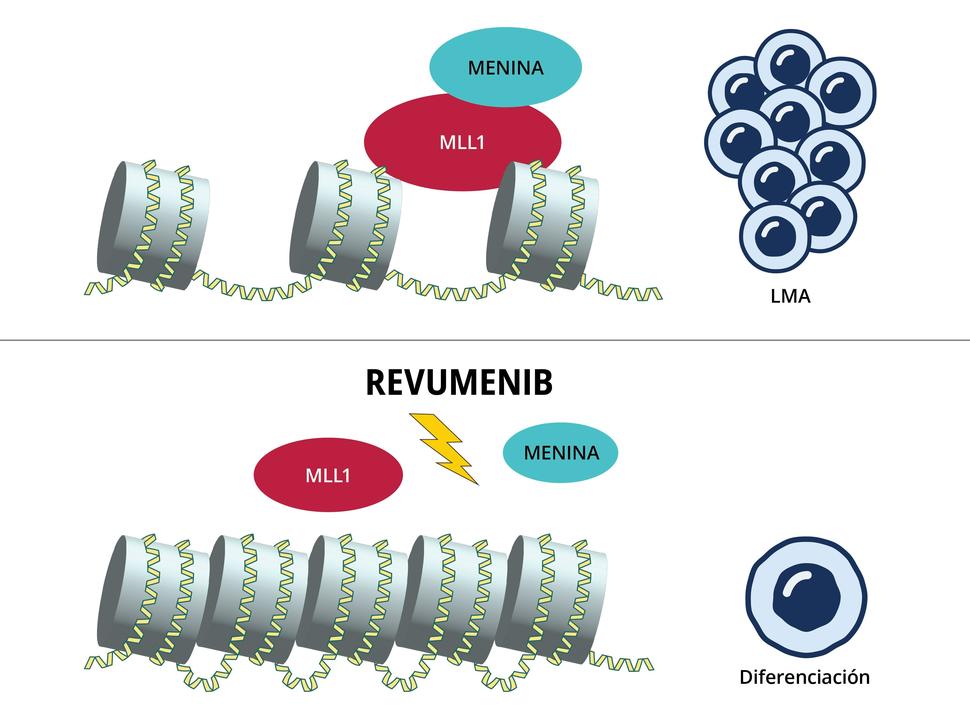
Figure 2. The MLL-protein complex recruits and binds to chromatin modifying complexes and histone methyltransferases that result in a promoted RNA polymerase II, which allows for productive elongation and activation of MLL-fusion target genes that cause differentiation block such as HOX, MEIS1, and BCL2, allowing for cells to proliferate.
VI. References
American Cancer Society. (2024). Key Statistics for Acute Myeloid Leukemia (AML). Retrieved from https://www.cancer.org/cancer/types/acute-myeloid-leukemia/about/key-statistics.html. Caslini, C., Yang, Z., El-Osta, M., Miline, T., Slany, R., Hess, J. (2007). Interaction of MLL Amino Terminal Sequences with Menin Is Required for Transformation. Cancer Research, 67(15), 7275-7283. DOI: 10.1158/0008-5472.CAN-06-2369 Kuhn, M., Armstrong, S. (2015).Designed to Kill: Novel Menin-MLL Inhibitors Target MLL-Rearranged Leukemia. Cancer Cell, 27(4), 431-433. DOI: 10.1016/j.ccell.2015.03.012 Matkar, S., Thiel, A., Hua, X. (2013). Menin: a scaffold protein that controls gene expression and cell signaling. Trends in Biochemical Sciences, 38(9), 394-402. DOI: 10.1016/j.tibs.2013.05.005 Murai, M., Chruszcz, M., Reddy, G., Grembecka, J., Cierpicki, T. (2011). Crystal Structure of Menin Reveals Binding Site for Mixed Lineage Leukemia (MLL) Protein. Journal of Biological Chemistry, 286(36), 31742-31748. DOI: 10.1074/jbc.M111.258186 Grembecka, J., Belcher, A., Hartley, T., Cierpicki, T. (2010). Molecular Basis of the Mixed Lineage Leukemia-Menin Interaction. Journal of Biological Chemistry, 285(52), 40690-40698. DOI: 10.1074/jbc.M110.172783 Reynolds, S. (2023). Revumenib Shows Promise in Treating Advanced Acute Myeloid Leukemia. National Cancer Institute. Retrieved from https://www.cancer.gov/news-events/cancer-currents-blog/2023/revumenib-menin-inhibitor-advanced-aml. Back to Top