Human METH-TAAR1 Complex
Skylar Helms '27 and Simone Morrow '25
Contents:
I. Introduction
Human trace-amine associated receptor 1 (TAAR1) recognizes a range of biogenic amines in the brain. This includes the endogenous β-phenylethylamine (β-PEA) as well as (S)-D-(+) methamphetamine (METH). TAAR1 is especially unique, having a widespread presence throughout the mammalian brain, along with expression in peripheral tissues related to energy metabolism and immune function; other TAARs are primarily expressed in olfactory regions. Due to its unique physiological role in the brain, TAAR1 is emerging as a promising target for a range of neurological disorders including schizophrenia, depression and drug addiction.
TAAR1 is a G-protein coupled receptor that interacts with a variety of molecules: β-PEA is the brain’s endogenous amphetamine, RO52566390, a synthetic compound selectively targeting TAAR1, and finally METH, which is an abused substance with no US FDA-approved medications to treat addiction.
II. General Structure
TAAR1 is a G-protein-coupled receptor (GPCR),
a heterotrimeric guanine nucleotide-binding protein that is made up of an alpha (Gα) beta (Gβ), and gamma (Gγ) subunit. Nb35 is a single-chain antibody that stabilizes the nucleotide-free complex by inhibiting the GTP-GDP exchange reaction by binding between the Gα and Gβ subunits. TAAR1 consists of canonical transmembrane domain (hydrophobic region of a protein that spans a cell membrane's lipid bilayer) of seven transmembrane helices (TM1–TM7) , all three extracellular loops 1–3 (ECL1–ECL3) , (located outside the cell and play a role in ligand recognition), a short intracellular loop 2 (ICL2) helix, and an amphipathic (containing both polar and nonpolar regions) helix, H8. Important to note is that ECL2 forms an additional helix that folds over the cap of the ligand-binding pocket.
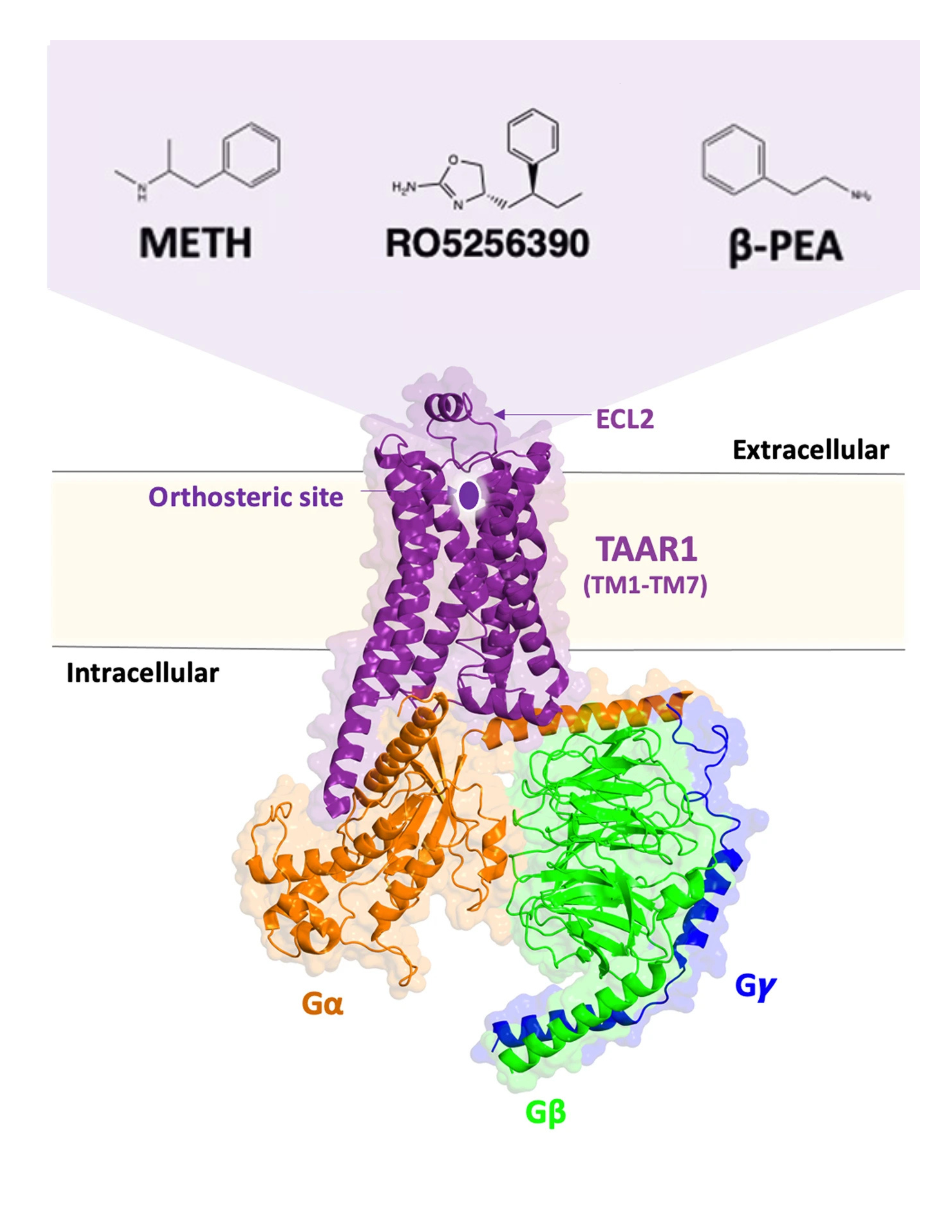
Figure 1. A type of G protein-coupled receptor, TAAR1, located intracellularly. Adapted from Nair et al. 2024.
III. METH-TAAR1 Binding Mode
METH occupies the orthosteric binding pocket of TAAR1. This pocket is lined by transmembrane helices 3, 5, 6, and 7, and capped by an ECL2 loop and the upper part of the ECL2 helix. TAAR1 recognizes METH through its two chemical moieties: a phenylpropyl group and an amino-methyl group. A notable polar interaction occurs between the secondary amine in the amino-methyl group and TAAR1 residue Asp103. Asp103 is further stabilized with Tyr294 and His99 through direct hydrogen bonds. Tyr294 forms a hydrogen bond with Ser80. This establishes a hydrogen-bonding network that consists of Asp103, Tyr294, Ser80, Arg83, and His99. The secondary amine in the amino-methyl group also forms a hydrogen bond with Ser107, stabilizing the ligand binding of METH in the TAAR1 pocket.
The phenylpropyl group of METH forms hydrophobic contacts. These include Ile104, Val184, Phe186, Thr194, Trp264, Phe267, and Phe268—hydrophobic residues that make up the binding pocket. The methyl group at the end of the propyl end is guided to a hydrophobic subpocket consisting of Phe267, Ile290, and Trp264. The methyl group located at the end of the amino-methyl group fits snugly in the hydrophobic environment closed in by Ile290 and Val184 (Val184 is in the ECL2 loop).
IV. β-PEA-TAAR1 Binding Mode
Trace amines are the endogenous agonists of TAAR1 . Due to its ability to induce amphetamine-like effects, especially at high doses, β-PEA is noticeable as the brain’s “endogenous amphetamine”. β-PEA has a similar structure to METH, but contains two less methyl groups.
β-PEA, like METH, also occupies the orthosteric binding pocket of TAAR1 . It is stabilized by polar interactions and a hydrogen-bonding network consisting of Asp103, Tyr294, Ser107, His99, Arg83, and Ser80. The phenyl group forms hydrophobic contacts with Phe186, Thr194, Trp264, Phe267, and Phe268.
β-PEA is 10 times more effective in activating TAAR1 than METH. This could be due to a shorter polar interaction with Asp103 along with the hydrogen bond with Ser107 (Asp103: 0.298 nm (2.98 Å), Ser107: 0.334 nm (3.34 Å)) in comparison to the binding interactions of METH (Asp103: 0.353 nm (3.53 Å), Ser107: 0.450 nm (4.50 Å)).
The absence of the hydrophobic methyl groups is likely what allows this change in distance to occur. Tyr294 is also able to interact directly with β-PEA , while METH’s interaction is through Asp103.
V. High Potency of RO5256390
Trace amines are the endogenous agonists of RO5256390, a clinical-stage drug, is both a potent and selective agonist of TAAR1, and has been found to alleviate various effects of psychiatric disorders, including suppressing cocaine-seeking behaviour and reducing relapse. RO5256390, is more potent than both METH and β-PEA. This is likely due to several interactions. First, the hydrophobic interaction between Val184 and Ile290 with the phenyl-butyl group. Second, the pi-pi interaction between Tyr294 and the oxazol ring. Finally, the hydrogen bond between Ser107 and 2-ylamine.
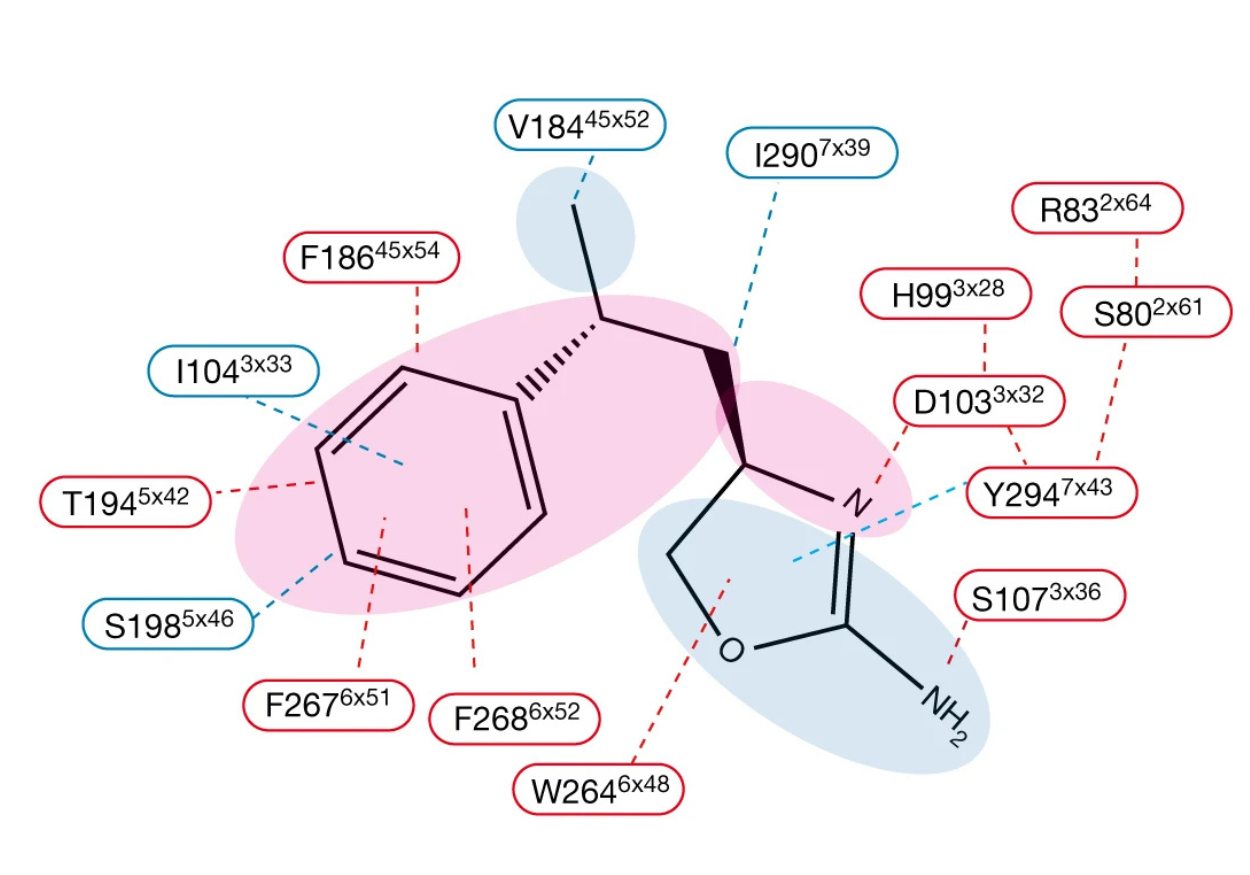
Figure 1. RO5256390 interactions with TAAR1 residues. The red color indicates common interactions seen with METH and β-PEA; blue indicates specificity of interactions for RO5256390 in its recognition by TAAR1. Reprinted from Liu et al. 2023.
VI. Medical Functions and Implications
TAAR1 has been shown to play a role in modulating METH’s neurobiological effects. Past studies have found the knockout of TAAR1 increases abuse-related behaviors from psychostimulants, while its activation helps reduce these behaviors. Due to these findings, TAAR1 has come forward as a promising target for addiction treatment. Its role in regulating not only METH, but also other psychostimulants has made it a target for treating not only addiction, but also other neuropsychiatric disorders, such as schizophrenia. Comparing the structures of TAAR1 bound to METH, the endogenous ligand β-PEA, and a clinical-stage drug, RO5256390, allows us to explore the common mechanisms of ligand-binding; the key residues contributing to ligand selectivity in TAAR1 can be compared among ligands. Currently, there are no US FDA-approved medications available to treat METH addiction (2023). The ability to discover targets present in METH’s signaling pathway, such as TAAR1, and develop an understanding of its mechanism of function is integral to the medical need of ameliorating psychostimulant addiction.
VI. References
Glyakina, Anna & Pavlov, Constantine & Sopova, Julia & Gainetdinov, Raul & Leonova, Elena & Galzitskaya, Oxana. Search for Structural Basis of Interactions of Biogenic Amines with Human TAAR1 and TAAR6 Receptors. International Journal of Molecular Sciences 23, 209. Hidetaka S. Oshima, Fumiya K. Sano, Hiroaki Akasaka, Aika Iwama, Wataru Shihoya, Osamu Nureki. 2024. Optimizing cryo-EM structural analysis of Gi-coupling receptors via engineered Gt and Nb35 application. Biochemical and Biophysical Research Communications 693: 0006-291X.
Liu, H., Zheng, Y., Wang, Y. et al. 2023.
Recognition of methamphetamine and other amines by trace amine receptor TAAR1. Nature 624:663–671.
Nair, P.C., Shajan, B. & Bastiampillai, T. 2024.
Newly identified structures of trace-amine associated receptor-1 (TAAR1) will aid discovery of next generation neuropsychiatric drugs. Molecular Psychiatry 29:1925–1928.
Back to Top