P. aeruginosa mercury(II)-bound Tn501 MerR
Slaybrina Raphael '26
Contents:
I. Introduction
* Use the dropdown list to change the protein representation throughout *
Mercury (Hg) readily accumulates in living organisms and is highly toxic due to its strong affinity for the thiolate groups found in various enzymes. The Tn501 mercury resistance operon in the Gram- negative bacterium Pseudomonas aeruginosa has been extensively studied. Its structural genes transport and transform both inorganic and organic mercury, thereby providing specialized mercury resistance. The mer operon is regulated by the mercury(II)-dependent transcriptional repressor-activator MerR. In the absence of Hg(II), MerR acts as a repressor. When bound to Hg(II), MerR acts as an activator.
II. General Structure
Tn501 MerR is a homodimer. Each monomer contains three distinct functional domains: an N-terminal DNA-binding domain (DBD, residues 1–81,
), a C-terminal mercury-binding domain (MBD, residues 119–134,
), and an amphipathic dimerization helix (DH, residues 82–118,
) that bridges the DBD and MBD3. Each DBD is structured in a positively charged helix-turn-helix motif (HTH)3. The
motif consists of helices α1 and α2, and the turn that connects them. α2-helix is the DNA-recognition helix that inserts into the major groove of promoter DNA3. The DH plays a crucial role in dimer stabilization by forming an antiparallel coiled-coil dimer interface. The functional domains primarily consist of α-helices and turns, and each domain participates in dimerization3. The accessible surface area for the
is about 4000Å23.
III. Mercury Binding
Each Tn501 MerR metal-binding domain (MDB) binds a single Hg(II) ion. The MBD consists of a
flexible metal binding loop (residues 119–127) and the remainder of the short two-turn
α6-helix (residues 128–134)
3. Each MBD packs against the
α4-helix of the other monomer and the
N-terminus of the dimerization helix .
contribute to Tn501 MerR Hg(II) binding
3.
Bound Hg(II) has planar trigonal coordination geometry with the Sulfur (S) atoms of three conserved cysteine residues:
Cys82’ (α4'),
Cys117 (α5), and
Cys126 (α6)
3
. All three Hg–S covalent bonds are about 2.56Å long. The bond angles deviate from the optimal 120° (118.0°, 114.9°, 127.0°)
3. This may be due to a steric effect caused by the pyrrolidine of
Pro127, which is packed against the
Cys117 Hg–S and
Cys126 Hg–S bonds
3
. His81', His118, Pro127, Leu128, and Ile129 are crucial for maintaining a suitable electrical charge for Hg(II) binding
3.
Cys126 is constrained by
Pro127, which allows it to form N–H…S hydrogen-bonding interactions with
Leu128 and
Ile129 on the polar two-turn helix. This helps neutralize the negative charge of Cys126’s thiolate anion
3. Without Pro127, MerR transcriptional activation is impaired
3. The negative charges in Cys82’ and Cys117 may be neutralized by the charge-charge interaction between
His81' and
His118 when one histidine is protonated
. When His118 is replaced with nonpolar alanine, MerR transcriptional activation is abolished
3
Figure 1:Example of an ion-dipole interaction between the deprotonated carboxyl and protonated amino groups of two histidine molecules4.
Cys82’ stabilizes the dimer and provides a higher coordination number optimal for Hg(II) binding
3. Planar trigonal coordination geometry with the three conserved cysteine residues allows Tn501 MerR to have a high affinity for Hg(II) (association constant ≈ 1047). Mutations of the coordinated cysteine residues dramatically decrease the affinity of MerR for Hg(II)
3.
Additionally, planar trigonal coordination geometry allows merR to distinguish Hg(II) from other metal ions
3. Other metal ions — such as Mn(II), Co(II), Ni(II), Pt(II), Cu(I), Tl(I) and Pb(II) — require different geometries. Thus, they are unable to activate mer operon transcription in vitro, even at high concentrations
3.
IV. DNA Binding
MerR binds DNA using 2 N-terminal DNA-binding domains structured in a winged helix-turn-helix motif. In the absence of mercury(II), MerR represses transcription of the mer operon by capturing RNA polymerase in a closed inactive state3. MerR binds an operator sequence in the −35 and −10 promoter spacer region as a dimer. At around 19 bp, the spacer region is longer than the optimal 17±1 bp, which hampers recognition by the σ70 subunit of RNA polymerase 1,2. When MerR binds, it causes the DNA to curve, allowing σ70 to interact with the −35 elements but not the -10 element, thus blocking transcription initiation1,2. When mercury(II) binds to MerR, the distance between the DNA recognition helices decreases. The conformational changes bend and twist the backbone, locally underwinding the promoter DNA by 33°1. This results in the re-alignment of the −35 and −10 elements and shortening of the spacer region to 17±1 bp. In turn, this allows optimal binding of the σ70 subunit, facilitating the formation of an open complex by RNA polymerase to initiate transcription3.
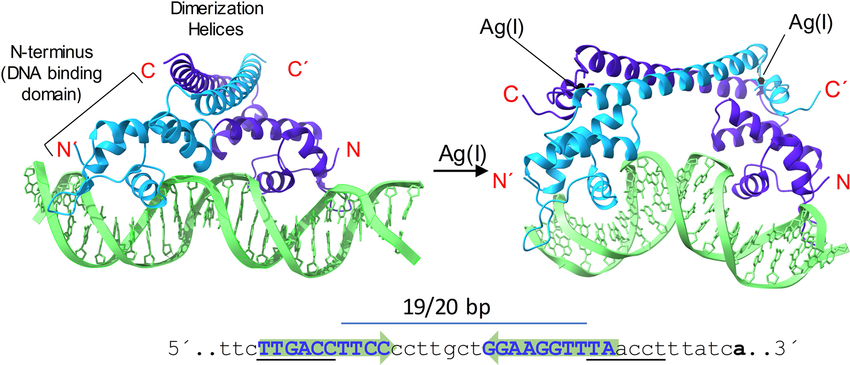
Figure 2: Example of the general transcription activation mechanism for a typical MerR regulator.2
V. Allosteric Signaling Network
When MerR binds Hg(II), a series of conformational changes occur, including the relocation of the MBD and a shift in the DBD3. Gly79 in the hinge region forms hydrogen-bonding interactions with Glu77, and with the MBD residues Ser125′and Cys126′ from the other monomer. The Oγ1 and Oγ2 atoms of Glu77 form a salt bridge and hydrogen-bond with the NH1 group and the Nε1 atom of residue Arg53, respectively3. The allosteric signal is continued as Arg53 on the DBD α3-helix forms hydrogen bonds with Leu32 on the DNA-recognition α2-helix 3. The chain continues as Leu32 bonds to Tyr27, which bonds with Arg25, which bonds with Glu22 3.
Previous mutagenesis experiments have identified several additional amino acids that are crucial for the ability of MerR to activate and repress transcription. Mutations in S125P, as well as G79S and R53W/R53Q, impair the repressive function of MerR 3. Mutations E72K and E84K create electrovalent and hydrogen bond interactions that favor the activated conformation when Hg(II) is absent3. Likewise, mutations L76F and A85V enhance the formation of hydrophobic interactions, which activate transcription in the absence of Hg(II)3
.
VI. References
1. Brown, N. L.; Stoyanov, J. V.; Kidd, S. P.; Hobman, J. L. The MerR Family of Transcriptional Regulators. FEMS Microbiology Reviews, 2003, 27, 145–163. https://doi.org/10.1016/s0168-6445(03)00051-2. 2. Tulin, G.; Figueroa, N. R.; Checa, S. K.; Soncini, F. C. The Multifarious MerR Family of Transcriptional Regulators. Molecular Microbiology, 2023, 121, 230–242. https://doi.org/10.1111/mmi.15212. 3. Wang, D.; Huang, S.; Liu, P.; Liu, X.; He, Y.; Chen, W.; Hu, Q.; Wei, T.; Gan, J.; Ma, J.; Chen, H. Structural Analysis of the Hg(II)-Regulatory Protein Tn501 MerR from Pseudomonas Aeruginosa. Scientific Reports, 2016, 6. https://doi.org/10.1038/srep33391. 4. Ye, M.; Li, H.; Zhang, Q.; Weng, Y.; Qiu, X. Intermolecular Hydrogen Bonds Formed Between Amino Acid Molecules in Aqueous Solution Investigated by Temperature-Jump Nanosecond Time-Resolved Transient Mid-IR Spectroscopy. Chinese Journal of Chemical Physics, 2007, 20, 461–467. https://doi.org/10.1088/1674-0068/20/04/461-467.
Back to Top