Biology Dept Kenyon College |
Microbial and Plant Development |

|
Biology Dept Kenyon College |
Microbial and Plant Development |

|
| Microbial
development
Plant development: Embryogenesis Plant development: Meristems Heterocyst formation in Anabaena and other cyanobacteria Cyanobacteria such as Anabaena grow as long filaments of photosynthetic vegetative cells. About every tenth cell, a vegetative cell differentiates into an anaerobic, nitrogen-fixing heterocyst. Heterocysts supply fixed nitrogen to neighboring vegetative cells in return for the products of photosynthesis. This separation of cellular functions is necessary because cyanobacteria have oxygen-evolving photosynthesis but the nitrogen-fixing enzyme, nitrogenase, is unstable in the presence of oxygen. The differentiation of heterocysts is provoked by an environmental cue, which is the absence of a fixed nitrogen source. The image below
shows filaments
of the cyanobacterium Anabaena. The arrow points to a
heterocyst.
Heterocysts are terminally differentiated cells that are highly specialized for nitrogen fixation. At least three programmed DNA rearrangements occur during heterocyst differentiation in Anabaena. The rearrangements involve the excision of DNA elements from the chromosome by site-specific recombination between short directly repeated sequences. Excision of two elements is shown below.
Genetic analysis of mutants that cannot differentiate properly has increased our understanding of the mechanism by which this differentiation occurs, as well as how the frequency of differentiating cells is regulated along each filament. One of the genes that has been shown to control heterocyst development is patS. Wild-type filaments (A) grown in complete medium and (B) in medium lacking nitrogen to induce heterocysts (arrowheads) are shown below. (C) Overexpression of the patS gene prevented heterocyst formation, and (D) deletion of the patS gene resulted in additional heterocysts with an abnormal pattern. Brackets indicate chains of contiguous heterocysts. Scale bars, 10 µm.
Fruiting body development in Myxococcus xanthus and other myxobacteria The myxobacteria
are an interesting
family of gliding bacteria that produce fruiting bodies in starvation
conditions.
Vegetative myxobacteria cells are elongated rods that glide across solid surfaces, secreting polysaccharide slime tracks in which cells migrate away from the colony edges (watch movie, also see panel A). When starvation conditions prevail and cell densities are above a threshold level, the cells migrate back along the slime tracks, aggregating by chemotaxis, to form large mounds of cells. These aggregates then develop into fruiting bodies (panels B and C, also closeup image) that are raised above the surface. As the vegetative cells migrate upwards into the fruiting body they undergo a progressive differentiation into spherical, thick-walled spores. A mixture of spherical spores and rod-shaped vegetative cells is shown in panel D.
Genetic
approaches are being
used to further our understanding of the mechanism of development in
myxobacteria.
A number of extracellular signals are necessary for fruiting body
development,
and mutants that have lost the ability to produce these extracellular
signals
have been isolated. These mutants are being used to dissect the genetic
program and to isolate and identify the signals.
Sporulation in
Bacillus
subtilis
Bacillus subtilis is a rod-shaped bacterium that grows and divides symmetrically in relatively rich media. However, when these bacteria encounter nutrient deprivation and dense population conditions, they undergo a developmental process called sporulation, which results in the production of resistant, metabolically inactive spores. Developing spores can be observed as the bright spheres shown in this image.
Sporulation involves the creation of two different cell types by asymmetric cell division. These two cells types have distinct programs of gene expression. The smaller daughter cell, the immature prespore cell, develops via a series of intermediate morphological stages into a highly resistant, dormant spore within the cytoplasm of the larger "mother cell".
Alternate sigma
factors play
a major role in the temporal and spatial control of gene expression
during
sporulation. The sequential expression of alternative sigma factors
allows
RNA polymerase holoenzyme to transcribe different subsets of promoters
at the proper times during development
Asymmetric cell division characterizes both Caulobacter crescentus differentiation (left) and Bacillus subtilis sporulation (right). 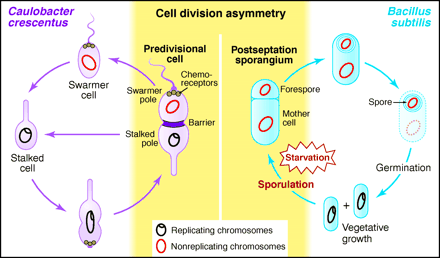 from Losick and Shapiro, Cell 276:712 - 718 (1997) Plant development is different from animal development. Because plant cells have rigid cell walls, plant cells can't migrate. Therefore, plant shape is based on the rate and direction of cell division and cell elongation. Although plants develop three basic tissue systems (dermal, ground, and vascular), they don't rely on gastrulation to establish this layered system of tissues.The flowering plant (angiosperm) life cycle is shown below, and the fertilization process is shown in more detail after that.
The egg cell and polar nuclei are contained within the embryo sac. The sperm nuclei are derived from the pollen grains.
Double fertilization results in a diploid zygote and a triploid endosperm, which provides nutrients to the developing embryo.
Plant embryogenesis begins with an asymmetric cell division, resulting in a smaller apical (terminal) cell and a larger basal cell. This first asymmetric division provides polarity to the embryo. Most of the plant embryo develops from the apical (terminal) cell. The suspensor develops from the basal cell. The suspensor anchors the embryo to the endosperm and serves as a nutrient conduit for the developing embryo. Further cell division leads to the globular stage. The three basic tissue systems (dermal, ground, and vascular) can be recognized at this point based on characteristic cell division patterns. The globular shape of the embryo is then lost as the cotyledons (embryonic leaves) begin to form. The formation of two cotyledons in dicots gives the embryo a heart-shaped appearance. In monocots, only a single cotyledon forms. Upright cotyledons can give the embryo a torpedo shape, and by this point the suspensor is degenerating and the shoot apical meristem and room apical meristem are established. These meristems will give rise to the adult structures of the plant upon germination. Further growth of the cotyledons results in the torpedo and walking-stick stages. At this point, embryogenesis is arrested, and the mature seed dessicates and remains dormant until germination.
from Susan Singer In the following
images,
the descendants of the apical cell are shown in yellow, and the
descendants
of the basal cell are shown in pink.
A large amount information on cell division patterns and organogenesis during embryo development has been accumulated based on descriptive studies. However, in order to reveal the mechanisms underlying the pattern formation during plant embryogenesis, one needs to experimentally perturb this process. Two approaches, experimental embryology and genetic dissection, have been used for this purpose. Because plant embryos are not easily accessible (they are developing within the ovule of the maternal parent), experimental embryology has relied on somatic embryogenesis - formation of embryos from adult cells in tissue culture . However, this approach is problematic since a high proportion of abnormal embryos occur quite often in tissue culture. In the past
decade, many
scientists have been attempting to genetically dissect the mechanisms
underlying
plant embryo pattern formation. This approach relies on the isolation
and
characterization of mutants which are defective in this process,
primarily
using the model plant Arabidopsis thaliana.
from Detlef Weigel
Mutants
have been
identified that result in changes in the establishment of the
apical-basal
pattern (organization of organs along the apical-basal axis) and
the radial pattern (organization of the three basic tissue systems - dermal,
ground, and vascular).
from Chun-Ming Liu Arabidopsis mutant seedlings were identified that showed a loss or distortion of the root, hypocotyl or cotyledon regions. These defects are presumed to result from defects during embryogenesis. These mutants were then placed into the following major classes: mutants lacking body segments along the apical-basal axis. This class includes gurke (gk), fackel (fk), monopterous (mp), and gnom (gn). mutants with disturbed radial symmetry - alterations of the radial pattern of tissue layers. This class includes knolle (kn) and keule (keu). mutants with disrupted organogenesis - these mutants have grossly abnormal overall shapes, but have all of the pattern elements along the apical-basal and radial axes. This class includes fass (fs), knopf (knf), and mickey (mic). Images of these
mutants are
shown below, with a wild type (wt) Arabidopsis seedling for
comparison.
from Jim Haseloff Arabidopsis mutants with defects in the apical-basal pattern can be further classified based on the PART of the seedling that is missing, analagous to the gap mutants of Drosophila.
from Jim Haseloff
The adult body of vascular plants is the result of meristematic activity. Plant meristems are centers of mitotic cell division, and are composed of a group of undifferentiated self-renewing stem cells from which most plant structures arise. Apical meristems are located at the growing tips of the adult plant, and produce root and shoot tissue. Shoot apical meristems (SAM) initiate leaves during vegetative development, and inflorescence (IM) and floral meristems (FM) during reproductive development.
This is a longitudianal section through a shoot apex. Compare this image to the diagram below.
image from Ross
Koning
This is a longitudianal section through a root tip. Compare this image to the diagram below.
How are shoot meristems organized? There are approximately 100 cells in the SAM of Arabidopsis thaliana. These cells are organized in two ways: cells are organized in radial zones and also in layers. Radial
organization:
Layer
organization:
The figure below
shows an
inforescence shoot apical meristem (SAM) and two adjacent floral
meristems
(FM) of Arabidopsis thaliana. On the left is the original laser
scanning confocal microscope optical section of tissue stained with
propidium
iodide to show the nuclei. The center image was colored to show radial
zonation within the SAM. The central zone
(CZ) is shown in red, the peripheral
zone (PZ) in green, and
the rib meristem (RM) in blue.
The image on the right was colored to show clonally-related layers.
The epidermal L1 layer is shown in
blue, the subepidermal
L2 layer is shown in pink,
and the L3 layer, or corpus
is shown in gold. The L1 and L2
together
are called the tunica.
from Elliot Meyerowitz
Throughout the plant's life, the meristem retains its size and shape, despite cell division and cell differentiation. What regulates the balance between cell differentiation and cell division? If cell differentiation were restricted, then the meristem would increase in size. In contrast, if cell division were restricted, then the meristem would decrease in size. Arabidopsis mutants that display altered shoot apical meristem structure have been identified. Shoot meristemless (stm) mutants are shoot meristemless - mutations in the STM gene completely block the initiation of the SAM during embryogenesis, but have no other obvious effects on embryo development. The image below shows a wild type seedling on the left, and an stm mutant seedling on the right. Notice the lack of a SAM between the cotyledons of the stm mutant.
Wuschel
mutants have
a flat SAM. One result of this mutation is the formation of flowers
with
fewer organs. The wuschel mutant flower on the right has only
one
stamen, and no central pistil. The wild type flower on the left has 6
stamens
and a central pistil.
image on left from Elliot
Meyerowitz
In contrast to shoot meristemless and wuschel mutants, clavatamutants have a much bigger shoot meristems than wild type plants, due to an overproliferation of cells in the SAM. Shown below are optical sections through the SAM of mature embryos stained with propidium iodide to show the nuclei. The clavata1 (clv1-4) mutant embryo on the right has a larger SAM with more cells than that of the wild type (left).
One result of clavata
mutations is the formation of extra floral organs from floral
meristems.
On the left is a wild type flower, and on the right, a clavata3
mutant flower with extra petals and stamens and an enlarged pistil.
|