Biology Dept Kenyon College |
|

|
| Plant development:
Embryogenesis Plant development: Meristems Plant and Animal development have in common:
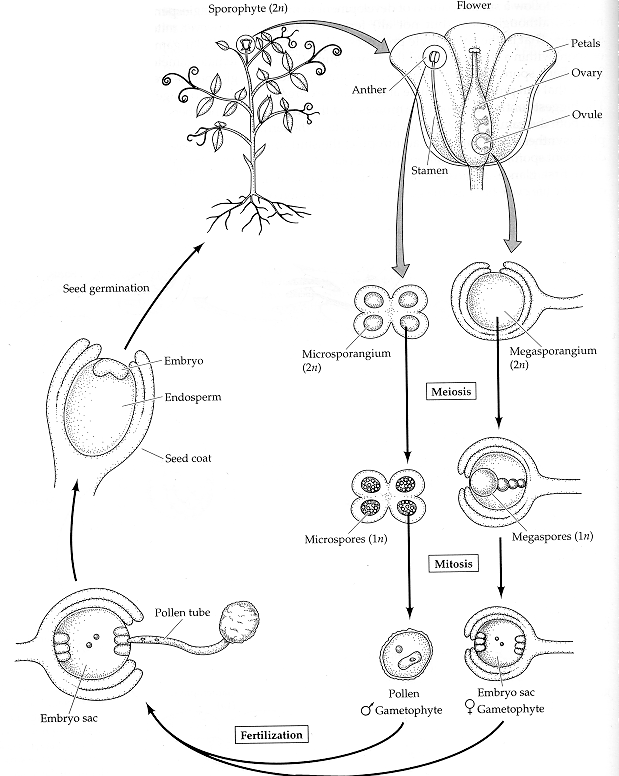
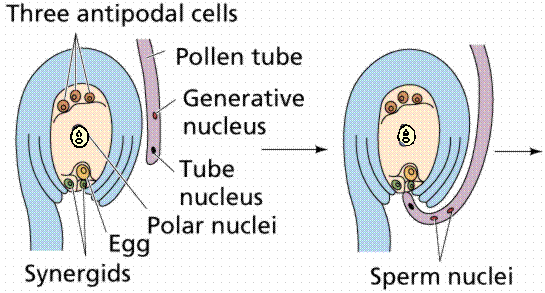
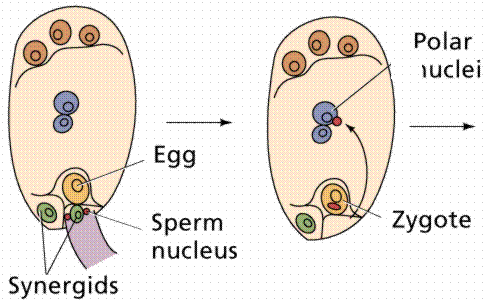
The egg cell and polar nuclei are contained within the embryo sac. The sperm nuclei are derived from the pollen grains. Double
fertilization.
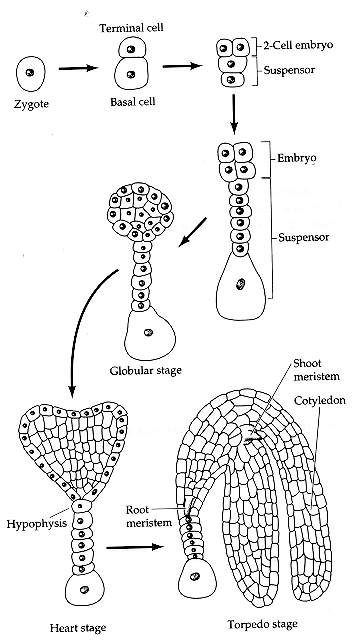
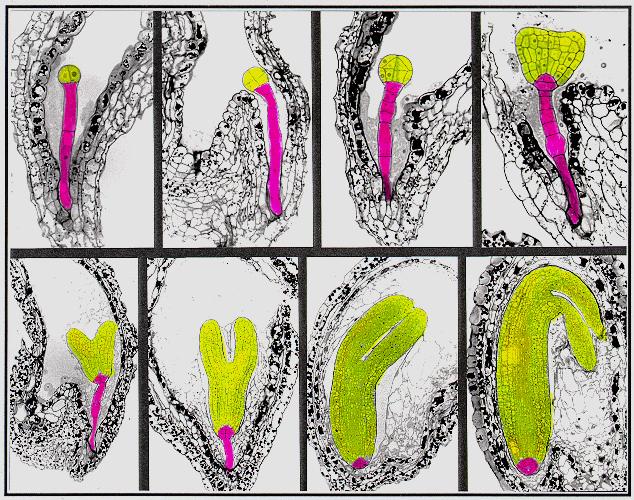
Plant embryogenesis begins with an asymmetric cell division, resulting in a smaller apical (terminal) cell and a larger basal cell. This first asymmetric division provides polarity to the embryo. Most of the plant embryo develops from the apical (terminal) cell. The suspensor develops from the basal cell. The suspensor anchors the embryo to the endosperm and serves as a nutrient conduit for the developing embryo. Further cell division leads to the globular stage. The three basic tissue systems (dermal, ground, and vascular) can be recognized at this point based on characteristic cell division patterns. The globular shape of the embryo is then lost as the cotyledons (embryonic leaves) begin to form. The formation of two cotyledons in dicots gives the embryo a heart-shaped appearance. In monocots, only a single cotyledon forms. Upright cotyledons can give the embryo a torpedo shape, and by this point the suspensor is degenerating and the shoot apical meristem and room apical meristem are established. These meristems will give rise to the adult structures of the plant upon germination. Further growth of the cotyledons results in the torpedo and walking-stick stages. At this point, embryogenesis is arrested, and the mature seed dessicates and remains dormant until germination.
In the following images, the
descendants of the apical cell are shown in yellow, and the descendants
of the basal cell are shown in pink.
A large amount information on cell division patterns and organogenesis during embryo development has been accumulated based on descriptive studies. However, in order to reveal the mechanisms underlying the pattern formation during plant embryogenesis, one needs to experimentally perturb this process. Two approaches, experimental embryology and genetic dissection, have been used for this purpose. Because plant embryos are not easily accessible (they are developing within the ovule of the maternal parent), experimental embryology has relied on somatic embryogenesis - formation of embryos from adult cells in tissue culture . However, this approach is problematic since a high proportion of abnormal embryos occur quite often in tissue culture. In the past decade, many
scientists have been attempting to genetically dissect the mechanisms
underlying plant embryo pattern formation. This approach relies on the
isolation and characterization of mutants which are defective in this
process, primarily using the model plant Arabidopsis thaliana.
from Detlef Weigel
Mutants have been
identified that result in changes in the establishment of the
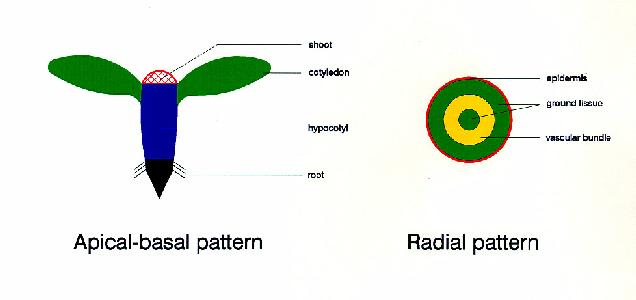
apical-basal pattern (organization of organs along the
apical-basal axis) and the radial pattern (organization of the three
basic tissue systems - dermal, ground, and vascular).
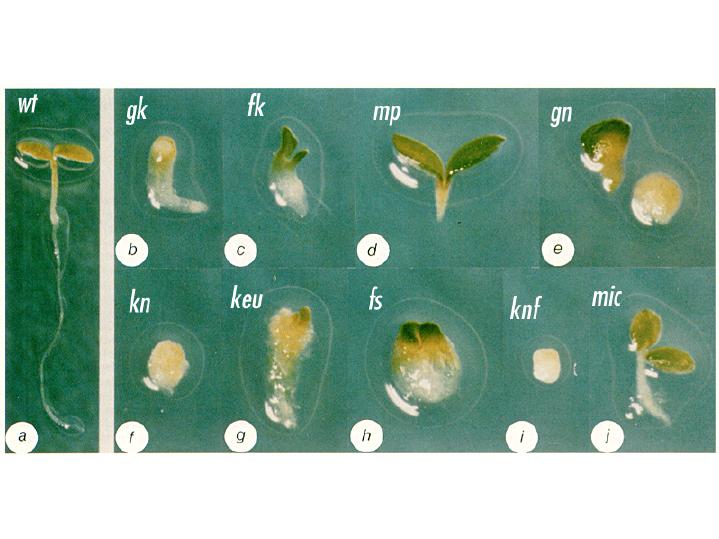
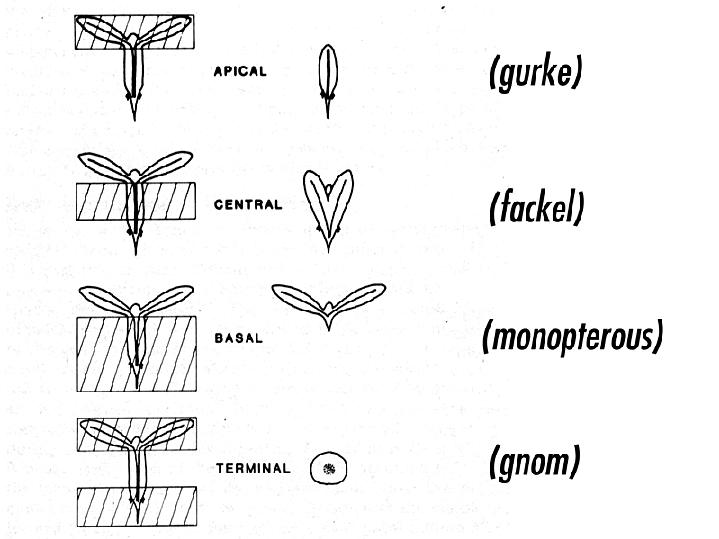
Arabidopsis mutant seedlings were identified that showed a loss or distortion of the root, hypocotyl or cotyledon regions. These defects are presumed to result from defects during embryogenesis. These mutants were then placed into the following major classes: mutants lacking body segments along the apical-basal axis. This class includes gurke (gk), fackel (fk), monopterous (mp), and gnom (gn). mutants with disturbed radial symmetry - alterations of the radial pattern of tissue layers. This class includes knolle (kn) and keule (keu). mutants with disrupted organogenesis - these mutants have grossly abnormal overall shapes, but have all of the pattern elements along the apical-basal and radial axes. This class includes fass (fs), knopf (knf), and mickey (mic). Images of these mutants are shown
below, with a wild type (wt) Arabidopsis seedling for
comparison. Arabidopsis mutants with defects in the apical-basal pattern can be further classified based on the PART of the seedling that is missing, analagous to the gap mutants of Drosophila.
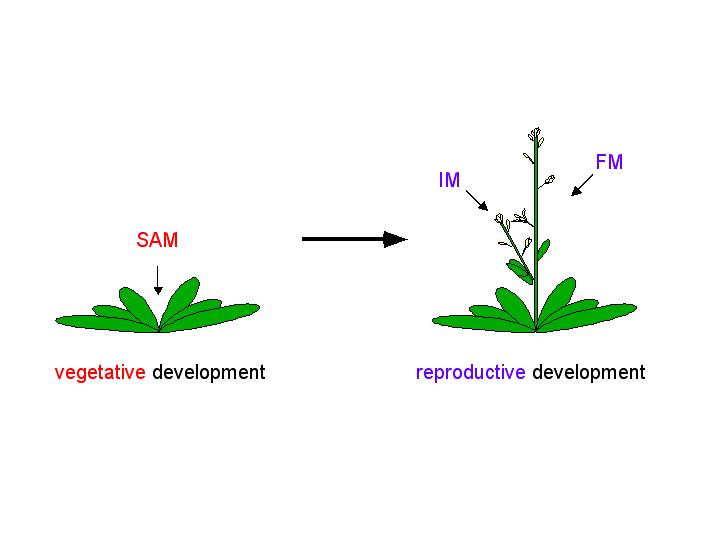
The adult body of vascular plants is the result of meristematic activity. Plant meristems are centers of mitotic cell division, and are composed of a group of undifferentiated self-renewing stem cells from which most plant structures arise. Apical meristems are located at the growing tips of the adult plant, and produce root and shoot tissue. Shoot apical meristems (SAM) initiate leaves during vegetative development, and inflorescence (IM) and floral meristems (FM) during reproductive development.
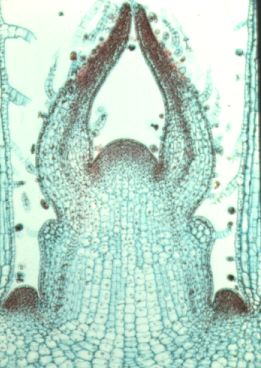
This is a longitudianal section through a shoot apex. Compare this image to the diagram below.
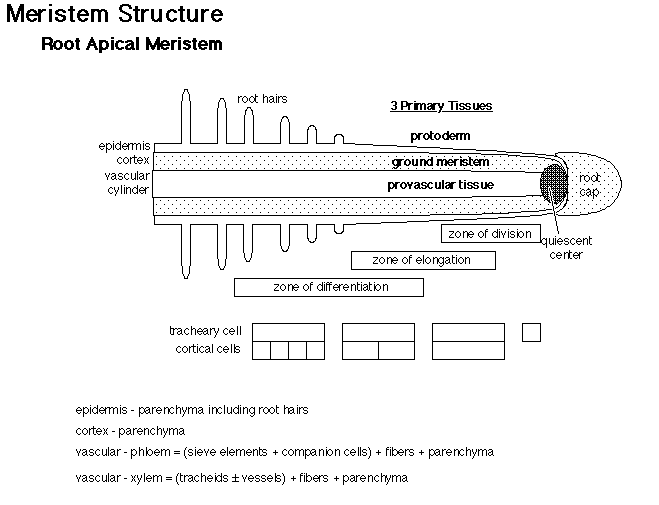
This is a longitudianal section through a root tip. Compare this image to the diagram below.
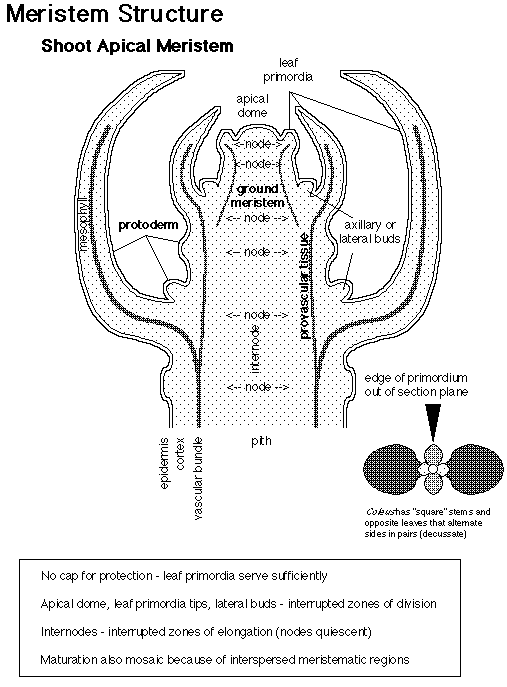
How are shoot meristems organized? There are approximately 100 cells in the SAM of Arabidopsis thaliana. These cells are organized in two ways: cells are organized in radial zones and also in layers. Radial organization:
Layer organization:
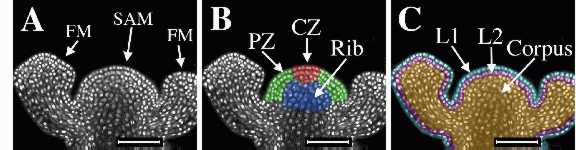
The figure below shows an
inforescence shoot apical meristem (SAM) and two adjacent floral
meristems (FM) of Arabidopsis thaliana. On the left is the
original laser scanning confocal microscope optical section of tissue
stained with propidium iodide to show the nuclei. The center image was
colored to show radial zonation within the SAM. The central zone (CZ) is shown in red, the peripheral
zone (PZ) in green, and
the rib meristem (RM) in blue. The image on the right was colored to
show clonally-related layers. The epidermal
L1 layer is shown in blue,
the subepidermal L2 layer is shown
in pink, and the L3 layer, or corpus
is shown in gold. The L1 and L2
together are called the tunica.
Throughout the plant's life, the meristem retains its size and shape, despite cell division and cell differentiation. What regulates the balance between cell differentiation and cell division? If cell differentiation were restricted, then the meristem would increase in size. In contrast, if cell division were restricted, then the meristem would decrease in size. Arabidopsis mutants that display altered shoot apical meristem structure have been identified. Shoot meristemless (stm) mutants are shoot meristemless - mutations in the STM gene completely block the initiation of the SAM during embryogenesis, but have no other obvious effects on embryo development. The image below shows a wild type seedling on the left, and an stm mutant seedling on the right. Notice the lack of a SAM between the cotyledons of the stm mutant.
Wuschel mutants have a flat SAM. One result of this mutation is the formation of flowers with fewer organs. The wuschel mutant flower on the right has only one stamen, and no central pistil. The wild type flower on the left has 6 stamens and a central pistil.
image on left from Elliot Meyerowitz
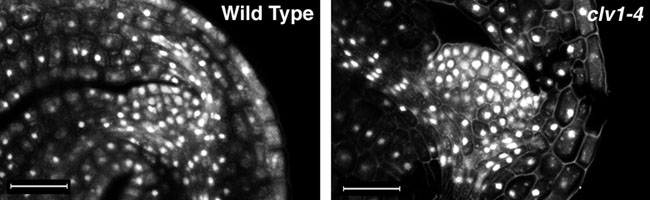
In contrast to shoot meristemless and wuschel mutants, clavatamutants have a much bigger shoot meristems than wild type plants, due to an overproliferation of cells in the SAM. Shown below are optical sections through the SAM of mature embryos stained with propidium iodide to show the nuclei. The clavata1 (clv1-4) mutant embryo on the right has a larger SAM with more cells than that of the wild type (left).
One result of clavata mutations is the formation of extra floral organs from floral meristems. On the left is a wild type flower, and on the right, a clavata3 mutant flower with extra petals and stamens and an enlarged pistil.
|