The quest to understand the mechanism by which plant cells perceive and transduce the ethylene signal is a difficult and ongoing challenge. Researchers have hypothesized that the ethylene molecule may interact with the plant cell by way of a membrane bound receptor. Further, it has been proposed that the specific interaction between the ethylene and the receptor protein may be mediated by a transition metal complexed to the receptor. (Bleeker and Schaller, 1996)

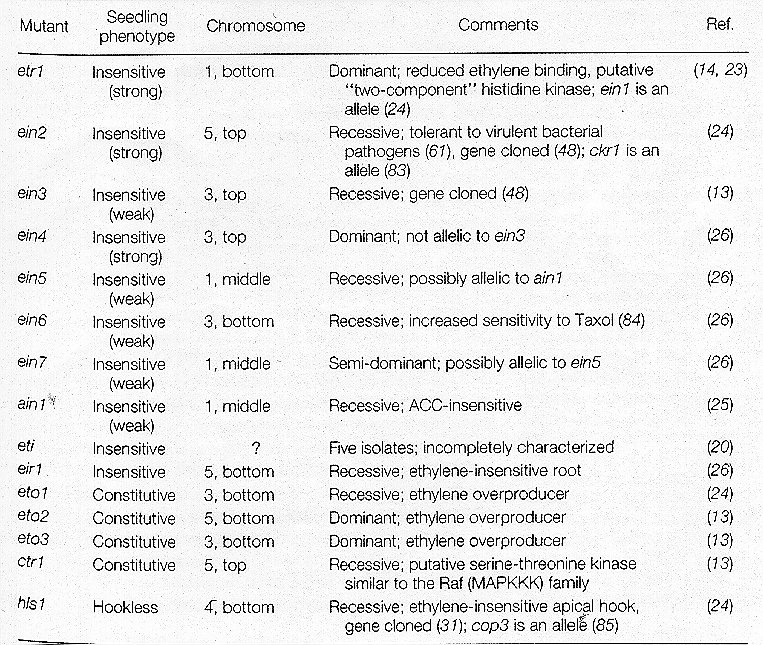

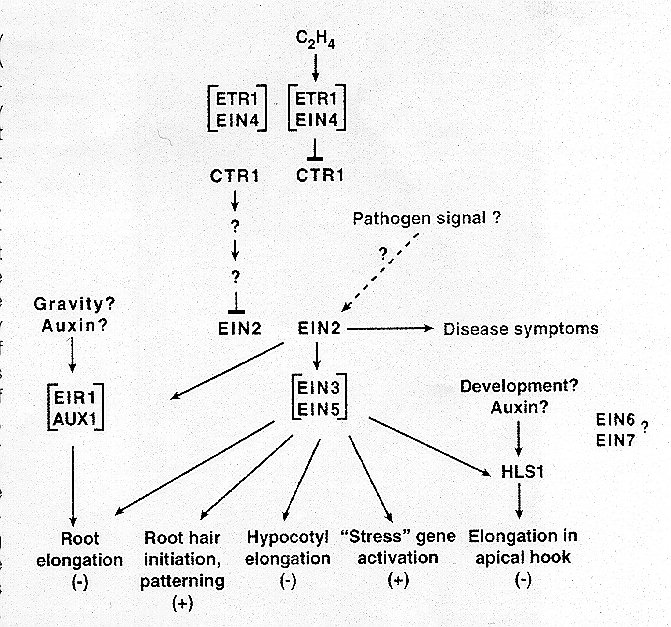
One of the genes identified by this method in Arabidopsis thaliana shows strong evidence of being an ethylene receptor. This gene has been named ETR1. This gene was identified by its mutant phenotype of being insensitive to ethylene. A second gene in the pathway was isolated, named CTR1, which was shown to cause constitutive ethylene response when mutated. CTR1 is epistatic to ETR1 and thus is thought to be a negative regulator of the ethylene response. (Bleeker and Schaller, 1996)
Interestingly, upon sequence analysis of the ETR1 and CTR1 genes, it is evident that the plant cell has incorporated two evolutionarily distinct signalling mechanisms into this pathway. First, the ETR1 amino acid sequence appears to be closely related to the two-component signal transduction pathway from bacteria, including the conservation of all residues required for His kinase activity. Secondly, the CTR1 sequence shows homology to the RAF-type Ser/Thr protein kinases from mammals. This could implicate a possible MAP kinase cascade involvement in ethylene signal transduction. From both these signalling mechanisms it is obvious that phosphorylation plays a large role in ethylene signalling. Additionally, the ethylene signal pathway as a whole seems to be similar in many ways to the osmosensing signal transduction pathway in yeast.(Bleeker and Schaller, 1996)
Through further mutational analysis and epistasis studies the following genetic pathway for ethylene action has been proposed:







The structure and function of the ETR1 gene product has been investigated in order to discover if and how it is functioning as a receptor for ethylene. The ETR1 protein has an N-terminal hydophobic domain, within which three potential transmembrane segments have been identified by hydropathy analysis. It is predicted that the N-terminus lies on the extracytoplasmic side of the membrane while the large C-terminus lies on the cytoplasmic side. Antibodies directed against ETR1 recognize a 79 kD protein in both Arabidopsis and transgenic yeast. However, analysis by SDS-PAGE in the absense of a reducing agent results in recognition of, primarily, a 147 kD protein, indicating that ETR1 exists as a disulfide linked dimer within the cell. The N-terminal has been implicated as important in dimerization.(Bleeker and Schaller, 1996)
One piece of evidence that strongly suggests the identity of ETR1 as an ethylene receptor is that plant cells containing a mutant allele (all mutants are dominant) have been shown to exhibit one-fifth of the saturable ethylene binding compared to wild type. Additionally, a crucial experiment was done using transgenic yeast. In this experiment transgenic yeast were produced which expressed the wild type ETR1 gene, these yeast were then shown to have saturable binding sites for [
The evidence that the ETR1 protein may contain a coordinatetransition metal which actually interacts with the ethylene molecule is purely theoretical as of yet. This type of interaction is well characterized in other systems, such as hemocyanin, hemoglobin, the oxygen sensing FixL protein in bacteria and the nitric oxide receptor in animals. If the ethylene molecule were to interact directly with the ETR1 protein it would have to be through some enzymatic reaction. This scenario, however, is not very likely because such an interaction would not be likely to be reversible, while it is known that ethylene is bound reversibly by the cell. If the ethylene were to interact with a transition metal (which was coordinated to the ETR1) the interaction would be of sufficiently low energy to be easily reversible. For these reasons it is postulated that a transitional metal coordination site (and thus the site of ethylene interaction) is formed perhaps by the three hydrophobic domains in the N-terminus or between monomer domains once the dimer is formed.(Bleeker and Schaller, 1996)
Once it is established that ETR1 (or another isoform) is in fact the ethylene receptor, the next question is, how does the receptor pass the signal on to downstream components? Bleecker and Schaller predict that "ethylene binding induces a conformational change within or between the subunits of the ETR1 dimer, consequently altering the rate of trans-phosphorylation between the His kinase domains." In light of this prediction they have devised two alternative models for the mechanism of signal transduction from the receptor, which are diagrammed below:

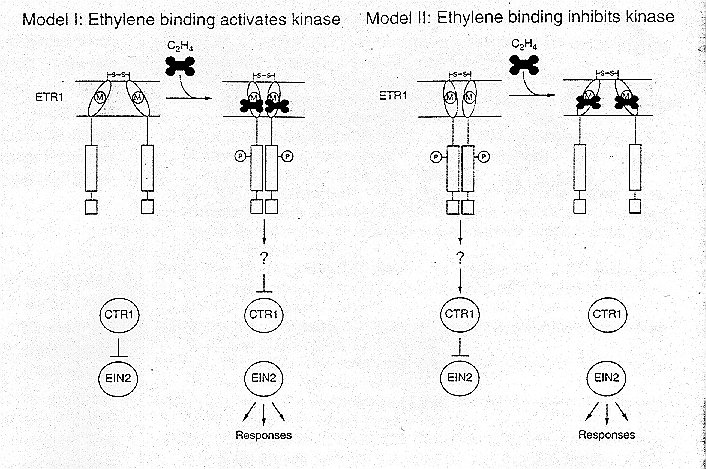

There is still a lot to be learned about this signal transduction pathway which is so central to so many functions in plants. Even when little pieces of the puzzle are elucidated it is still an enormous job to figure out how they fit together into the complex functioning of the plant. The tools of molecular biology have greatly sped up, in recent years, the process of decoding this mystery but there is still a long way to go.
 Return to main page
Return to main page