 Most plants cannot survive periodic flooding. Those that can survive react with growth responses that essentially separate them from areas of flooded soil and water. Plants that
cannot grow up out of the water, change their roots, or otherwise return themselves to an environment in which they are not surrounded by water, generally die. As water level in
soil rises, the amount of oxygen available for plant roots and for organisms living in the soil decreases (Laan et al. 1989b in Blom et al. 1996). Hypoxia tends to develop as soil
microorganisms consume what little oxygen is left in the soil, and oxygen diffusion from the surface slows. Nitrification rate is inhibited, and toxic compounds are often formed as
anaerobic bacteria use nitrate, manganese, and sulfate as alternative electron acceptors (Patrick and Mahapatra, 1968, Reddy et al. 1989). In areas with periods of flooding and
drydown, soil particles can settle between floods, increasing the mechanical resistance of the soil to growing roots (Blom et al. 1996).
Most plants cannot survive periodic flooding. Those that can survive react with growth responses that essentially separate them from areas of flooded soil and water. Plants that
cannot grow up out of the water, change their roots, or otherwise return themselves to an environment in which they are not surrounded by water, generally die. As water level in
soil rises, the amount of oxygen available for plant roots and for organisms living in the soil decreases (Laan et al. 1989b in Blom et al. 1996). Hypoxia tends to develop as soil
microorganisms consume what little oxygen is left in the soil, and oxygen diffusion from the surface slows. Nitrification rate is inhibited, and toxic compounds are often formed as
anaerobic bacteria use nitrate, manganese, and sulfate as alternative electron acceptors (Patrick and Mahapatra, 1968, Reddy et al. 1989). In areas with periods of flooding and
drydown, soil particles can settle between floods, increasing the mechanical resistance of the soil to growing roots (Blom et al. 1996).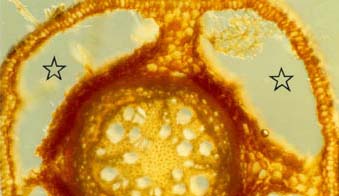 In response to these changes in soil chemistry and structure, hydrophytes alter their root systems when in submerged soils. Plant cells need oxygen, whether they are
photosynthetic or not, and root cells require oxygen in order to divide and grow. To ensure transport of oxygen to roots, flood-resistant plant species partially replace their root
systems with aerenchymous tissue when flooded. These new roots are characterized by aerenchyma formed by cell differentiation and collapse (lysigenous aerenchyma) or by cell
separation without collapse (schizogenous aerenchyma) (Justin and Armstrong 1987, Blom et al. 1996). This forms large continuous air spaces that allow diffusion of oxygen from
shoot to root. Of course, this system works best in plants that are also able to elongate their shoots during flooding, in order to raise leaves above the water level. A build-up of
ethylene is thought to contribute to this shoot elongation (Blom et al. 1996). Plants such as Rumex spp. can replace more than 20% of their old roots with aerenchymous root after
just two weeks of flooding. There appears to be a genetic component to flood resistance, since in this same study Rumex plants from low-lying areas had longer aerenchymous roots
than Rumex from drier habitats (Blom et al. 1996).
In response to these changes in soil chemistry and structure, hydrophytes alter their root systems when in submerged soils. Plant cells need oxygen, whether they are
photosynthetic or not, and root cells require oxygen in order to divide and grow. To ensure transport of oxygen to roots, flood-resistant plant species partially replace their root
systems with aerenchymous tissue when flooded. These new roots are characterized by aerenchyma formed by cell differentiation and collapse (lysigenous aerenchyma) or by cell
separation without collapse (schizogenous aerenchyma) (Justin and Armstrong 1987, Blom et al. 1996). This forms large continuous air spaces that allow diffusion of oxygen from
shoot to root. Of course, this system works best in plants that are also able to elongate their shoots during flooding, in order to raise leaves above the water level. A build-up of
ethylene is thought to contribute to this shoot elongation (Blom et al. 1996). Plants such as Rumex spp. can replace more than 20% of their old roots with aerenchymous root after
just two weeks of flooding. There appears to be a genetic component to flood resistance, since in this same study Rumex plants from low-lying areas had longer aerenchymous roots
than Rumex from drier habitats (Blom et al. 1996).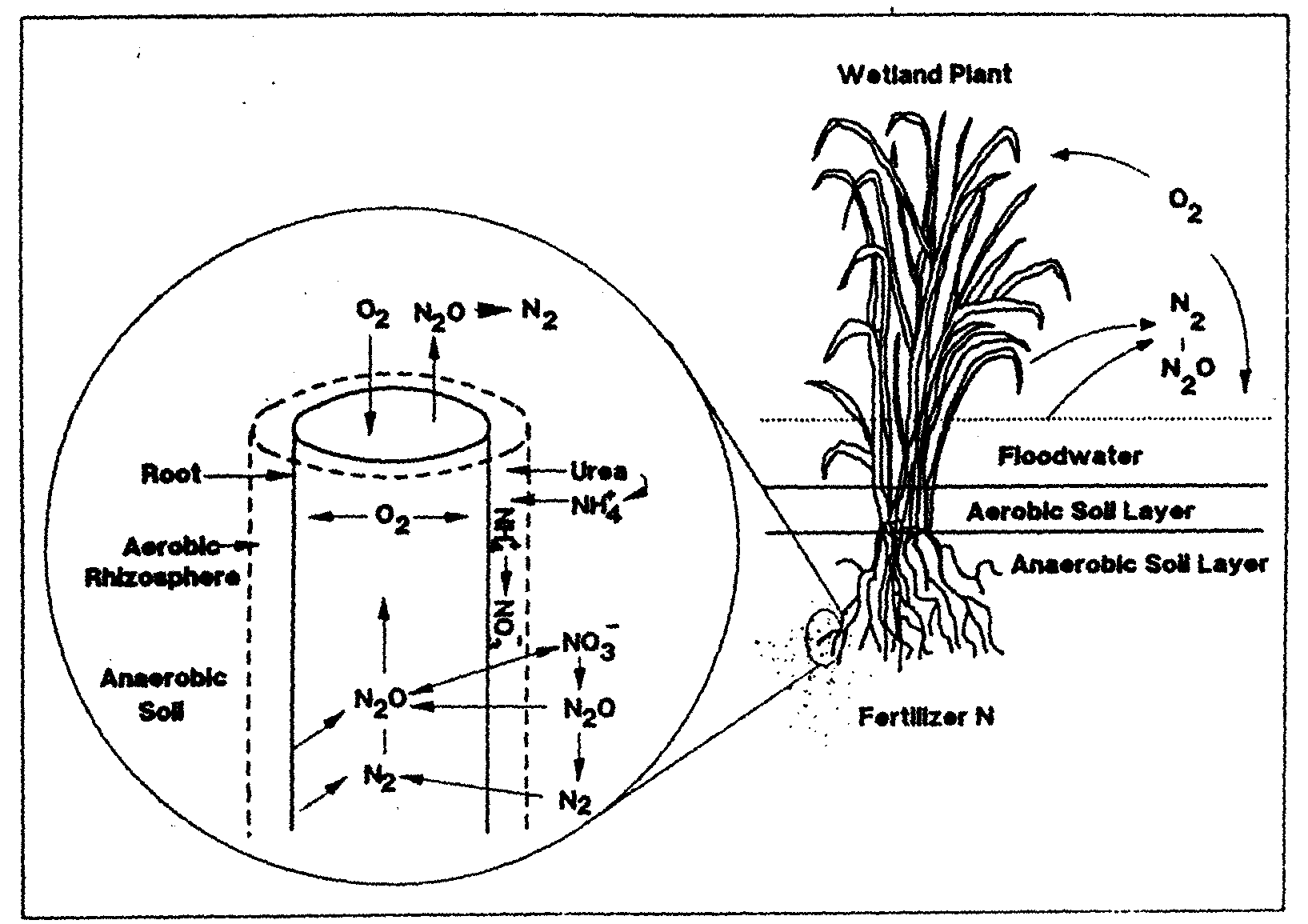 Some of the oxygen transported through the aerenchyma to plant root tips leaks out of pores in the root (pores result from aerenchyma formation) and into the surrounding soil.
This can result in a small zone of oxygenated soil around individual roots, called the oxidized rhizosphere (Armstrong and Armstrong 1988 in Blom et al. 1996). Oxygen-depleted
soils can reach almost original oxygen concentrations around plant roots (Blom et al. 1996). This oxidized rhizosphere allows normal root metabolism to occur, even in flooded soils
(Engelaar et al. 1991, 1993a in Blom et al. 1996). The absorption of toxic manganese and sulfur compounds which build up in anaerobic sediments is partially blocked by the
oxidized rhizosphere, which provides a zone around roots in which toxic compounds can be oxidized into less harmful forms. The oxidized rhizosphere also has a major effect on the
two nutrients that most often limit hydrophyte growth, phosphorus (P) and nitrogen (N) (Barko et al. 1991).. Hydrophytes have a second potential source for phosphorus and
nitrogen uptake, the surrounding water, but take up the majority of their P and N from aquatic sediments (Barko et al. 1991). Nutrient uptake in aquatic sediments is often limited
by the slow rate of N and P diffusion over low-density organic sediments as opposed to terrestrial soils (Drew and Lynch 1980 in Barko et al. 1991, Patrick and Mahapatra, 1968).
In normal aquatic sediments, this slow rate of diffusion is counteracted by the higher amounts of soluble N and P compounds in the soil, but this is not the case in the oxidized
rhizosphere. Aerenchymous roots provide root cells with much-needed oxygen, but can rob them of nutrients by forming insoluble phosphorus and nitrogen compounds when excess
oxygen leaks from the roots (Tessenow 1978, Baynes 1978 in Barko et al. 1991). The hydrophyte Hydrilla verticillata, for example, was found to reduce sediment nitrogen
concentrations by more than 90%, and phosphorus concentrations by more than 30%, over a matter of weeks (Barko et al. 1988 in Barko et al. 1991). Transport of nutrients to
shoots and leaves of hydrophytes also contributes to sediment nutrient depletion, at least until the plants later decompose. Since nutrient levels can play a major role in determining
the extent of VA mycorrhizal infection in terrestrial plants, and under some conditions can even result in parasitic rather than mutualist infection , it seems likely that nutrient levels
play a similarly important role in determining infection in hydrophytes (Johnson 1993).
Some of the oxygen transported through the aerenchyma to plant root tips leaks out of pores in the root (pores result from aerenchyma formation) and into the surrounding soil.
This can result in a small zone of oxygenated soil around individual roots, called the oxidized rhizosphere (Armstrong and Armstrong 1988 in Blom et al. 1996). Oxygen-depleted
soils can reach almost original oxygen concentrations around plant roots (Blom et al. 1996). This oxidized rhizosphere allows normal root metabolism to occur, even in flooded soils
(Engelaar et al. 1991, 1993a in Blom et al. 1996). The absorption of toxic manganese and sulfur compounds which build up in anaerobic sediments is partially blocked by the
oxidized rhizosphere, which provides a zone around roots in which toxic compounds can be oxidized into less harmful forms. The oxidized rhizosphere also has a major effect on the
two nutrients that most often limit hydrophyte growth, phosphorus (P) and nitrogen (N) (Barko et al. 1991).. Hydrophytes have a second potential source for phosphorus and
nitrogen uptake, the surrounding water, but take up the majority of their P and N from aquatic sediments (Barko et al. 1991). Nutrient uptake in aquatic sediments is often limited
by the slow rate of N and P diffusion over low-density organic sediments as opposed to terrestrial soils (Drew and Lynch 1980 in Barko et al. 1991, Patrick and Mahapatra, 1968).
In normal aquatic sediments, this slow rate of diffusion is counteracted by the higher amounts of soluble N and P compounds in the soil, but this is not the case in the oxidized
rhizosphere. Aerenchymous roots provide root cells with much-needed oxygen, but can rob them of nutrients by forming insoluble phosphorus and nitrogen compounds when excess
oxygen leaks from the roots (Tessenow 1978, Baynes 1978 in Barko et al. 1991). The hydrophyte Hydrilla verticillata, for example, was found to reduce sediment nitrogen
concentrations by more than 90%, and phosphorus concentrations by more than 30%, over a matter of weeks (Barko et al. 1988 in Barko et al. 1991). Transport of nutrients to
shoots and leaves of hydrophytes also contributes to sediment nutrient depletion, at least until the plants later decompose. Since nutrient levels can play a major role in determining
the extent of VA mycorrhizal infection in terrestrial plants, and under some conditions can even result in parasitic rather than mutualist infection , it seems likely that nutrient levels
play a similarly important role in determining infection in hydrophytes (Johnson 1993). Nutrient levels, however, do not most severely limit the distribution and spread of VA mycorrhizae in hydrophytes. Oxygen concentration is thought to play a larger role in determining VA mycorrhizal distribution in aquatic sediments. VA mycorrhizae are assumed to be obligate aerobes, although as Andre Cantelmos and Joan Ehrenfeld most recently pointed out, this assumption has never been tested (1999). Read and Armstrong (1972) came closest to explicitly testing this hypothesis, but their conclusions about ectomycorrhizae may not apply to VA mycorrhizae. If VA mycorrhizae can only survive in oxygenated environments, it was thought, they cannot grow in completely flooded soils. Several researchers have shown that this is not the case, that hydrophytes in submerged soils can develop substantial mycorrhizal infection (Keeley 1980, Cooke and Lefor 1998, Fennessy and Marx unpublished data 1999). Yet mycorrhizal infection varies vertically in submerged soils (Cooke et al. 1993). If mycorrhizae do not need oxygen to survive, why are they limited to certain areas of submerged hydrophyte roots? The few studies that have addressed these questions suggest that VA mycorrhizae may be dependent on the presence of an oxidized rhizosphere, but not necessarily confined by its area (Keeley 1980, Cantelmos and Ehrenfeld 1999). The range of oxygen concentrations in which VA mycorrhizae can survive, and whether or not such a range limits their distribution, is unknown. Knowing whether or not VA mycorrhizae are constrained by the oxidized rhizosphere will help answer important questions about the role of VA mycorrhizae in hydrophyte community dynamics. For a description of some of the research examining these questions, see Where do we go from here?