Biomorph
Challenge #4 |
Marine Biology: Animals and Ocean
| 1. The Biomorphs reassure you: "Our technology will add only 0.0001 moles/liter of hydrogen ions to your ocean. This tiny amount will have no effect on the ecosystem." What do you say? A concentration of 0.0001 moles/liter may look small, but it adds acidity equivalent to -log(0.0001M) = pH 4. This pH is acidic and will dissolve all calcium carbonate in the shells of marine animals. There will be no more coral, and many algal producers will go extinct. |
|
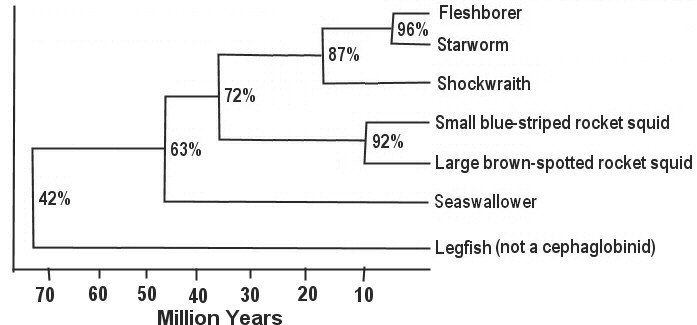
2. The evolution of cephaglobinids of Shora is measured by a "molecular clock" gene whose sequence varies by mutation at 0.8% change per million years. The following table shows the percent relatedness of all pairs of species. Based on this table, draw a phylogenetic tree showing divergence with time (on the x axis).
Draw map using these divergence times: (100 42)% / 0.8 % change per million years = 72.5 million years (100 63)% / 0.8% = 46.3 million years (100 72)% / 0.8% = 35 million years (100 87)% / 0.8% = 16.3 million years (100 92)% / 0.8% = 10 million years (100 96)% / 0.8% = 5 million years
3. The pH of Shoras ocean is 8.35. (a) Calculate the concentration of H+ in the water. [H+] = 10-8.35 = 4.47 x 10-9 moles/liter (Molar) (b) Is this pH good for the "raft corals"? Explain why or
why not. 4. As CO2 dissolves in water, some of it converts to carbonic
acid, which releases hydrogen ions. Write the chemical equation
for these processes.
The
Henderson-Hasselbalch equation is: pH = 6.1 + log(30) = pH 7.58. This pH value is way too high above 7.4; may cause convulsions. [Too low would cause coma.]
(a) Estimate the percent ionization (dissociation) of solutions with
the following concentrations (moles/liter) of carbonic acid. Assume
that only a tiny amount of carbonic acid is dissociated, and that no
other acids contribute hydrogen ions: For 0.0001 M carbonic acid (H2CO3): Ka = 9.3 x 10-7 = [H+][HCO3-] / [ H2CO3] = x2 /(0.01 M x) Assume
most carbonic acid stays protonated: x = sqrt (9.3 x 10-11) = 9.64 x 10-6 moles/liter pH = 5.02 % ionization = (amt ionized/original) x 100 = (9.64 x 10-6M / 10-4M) x 100 = 9.6%
For 0.001 M carbonic acid (H2CO3): Ka = 9.3 x 10-7 = [H+][HCO3-] / [ H2CO3] = x2 /(0.001 M x) Assume
most carbonic acid stays protonated: x = sqrt (9.3 x 10-10) = 3.05 x 10-5 moles/liter pH = 4.51 % ionization = (bicarbonate ion /original) x 100 = (3.05 x 10-5M / 10-3M) x 100 = 3.05%
For 0.01 M carbonic acid (H2CO3): Ka = 9.3 x 10-7 = [H+][HCO3-] / [ H2CO3] = x2 /(0.01 M x) Assume
most carbonic acid stays protonated: x = sqrt (9.3 x 10-9) = 9.64 x 10-5 moles/liter pH = 4.02 % ionization = (amt ionized/original) x 100 = (9.64 x 10-5M/ 10-2 M ) x 100 = 0.96%
(b) Calculate the pH at each concentration.
Comment on the trend you see in your results. What does it imply for
the effect of increasing Earth's atmospheric CO2 on marine
life? For
0.0001 M, x
= sqrt (9.3 x 10-11) = 9.64 x 10-6 moles/liter
pH = 5.02 Trends: as the concentration of the acid increases, the pH decreases, and therefore acidity (H+ concentration) increases. The trend shows how increasing CO2 dissolving as carbonic acid will acidify Earth's ocean.
(a) Microbes cooperate with other organisms: Explain an example from Shora, and an example from real life. Explain why the cooperation is favored by natural selection. On Shora, breathmicrobes in human skin help Sharers breathe under water. The breathmicrobes gain nutrients and protection from predators. Both partners gain more than they could apart; therefore, the mutualism is favored by natural selection. Algae within a predatory paramecium photosynthesize, providing nutrients for their host, which protects them from predation. The mutualism is favored, so long as light is available for photosynthesis and the paramecium is well fed. When the paramecium starves, it may digest the algae. Human digestive bacteria contribute 15% of our caloric uptake. The bacteria receive nutrients and protection. The mutualism is favored so long as the human is well fed. (b) Large animals and/or plants cooperate with each other: Explain an example from Shora, and an example from real life. Explain why the cooperation is favored by natural selection. On Shora, rafts harbor nests of fleshborers that attack seaswallowers, thereby protecting the raft. The fleshborers receive a secure home on the ocean, and a supply of food (fish feeding on the raft). The system is favored because each partner benefits. Tick birds feed on parasites on the hides of animals such as buffalo or rhinoceros. Similarly, wrasses feed on parasites from larger fish. The partners tolerate each other because the one receives nutrition whereas the other is rid of energy-draining parasites. Plants such as the acacia tree provide a home and nutrition for ants that protect the tree from browsing animals. The system is favored so long as animals are trying to browse the tree. Without browsing, the plants provide less support for the ants. |