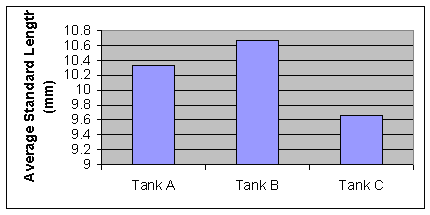
Figure 1: Average Standard Length of first week (Nov. 1) of measurements. (F=2..33, P=0.178)
The Effect of Temperature on Growth Rate of Juvenile Hippocampus Zosterae.
Adrian Nelson
Independent Study with Prof. Fennessey
1. Introduction
The decline in wild populations of seahorses, due to the medicinal and hobby trades, has led to increased research
on the part of aquaculturists to protect these species. Seahorse aquaculture has great potential in that the demand
for seahorses is greater than the supply, with at least 20 million seahorses traded globally in 1995 (Vincent,
1996). With the possibility of aquaculturing captive-bred seahorses to supply this demand, it can be both profitable
for investors, as well for the conservation of the wild populations, making this into an eco-friendly business.
Most species of seahorses are exploited in the Indo-Pacific region where harvesting protocols are few and far between.
For conservation to be successful there must be enough of a financial incentive for local fishermen to switch to
aquaculturing techniques. These techniques must be inexpensive and simple enough to allow local fishermen to permanently
shift to aquaculture and keep their independent status instead of having to be funded by outside companies. An
inexpensive method to increase the growth rate of seahorses to marketable size is needed to keep supply up with
demand. It is well known that an increase in temperature leads to an increase in growth rate for fish (James, Woods,
2001). This study will measure the maximum rearing temperature for optimum growth and survival of the seahorse
Hippocampus Zosterae.
Hippocampus Zosterae is a perfect candidate for aquaculture for many reasons. Firstly, they are not territorial
or cannibalistic, and so they can be raised in relatively high stocking densities. Secondly, they are known to
be one of the hardiest species of all Seahorses (Strawn, 1958). Thirdly, their natural growth rate allows them
to reach marketable size within 5 months, allowing 2 marketable periods a year.
The oceanic seahorse, Hippocampus Zosterae (Dwarf, Pygmy Seahorse), is widely distributed throughout the coral
reefs and grassy beds of the Gulf of Mexico, and the Caribbean. Dwarf seahorses live within depths of 3 meters
and live in temperatures of 20 - 25ºC. Although one of the smallest of all seahorses reaching a maximum size
of 2 in. the Dwarf Seahorse is one of the most hardy to rear (Strawn, 1958). Hippocampus Zosterae is considered
vulnerable on the CITES listing of worlds most endangered species (CITES, 2001).
2. Materials and Methods
Three 10-gallon tanks, A, B, and C were used for this experiment. Salt water was produced by adding 30 mg Instant
Ocean per gallon of aged de-ionized tap water. Activated Carbon with a Whisper filtration system was used in each
tank. Pebbles were used as a substrate and artificial plants were used as holdfasts for the seahorses. Light was
produced by 5, 60 watt fluorescent lights, 3 on cycles of (10hr/day, 14 hr/night), and the other two on cycles
of (12hr/day, 12hr/night) to give the effects of dawn and dusk. Seahorses were fed to excess with live Artemia,
enriched with SELCO, 3 times daily at 12 pm, 6 pm, and 12 am for a total of 35 days.
By basic aquarium heaters, the water temperatures in tanks A, B, and C were 30 ºC, 25 ºC, and 20 ºC
± 2 ºC, respectively. pH values were measured 3 times a week and ammonia and salinity were measured
biweekly. Values for pH in tank A were 8.3 ± 0.2, B 8.46 ± 0.05, and C 8.35 ± 0.15, ammonia
was 0.325 ± 0.20, and salinity 1.019. 25% water changes were done biweekly.
One pregnant male and 3 mated pairs of Hippocampus Zosterae were purchased from
Seahorse Farms USA. 10 dwarf seahorses were born from the pregnant male. Four were placed in tank A 30 ºC,
three in tank B 25 ºC, and three in tank C 20 ºC. Standard measurements of the seahorses, from crown
to tail, were measured in mm and done once a week by hand.
3. Data Analysis
The rate of growth for Hippocampus Zosterae was analyzed using a one-way ANOVA for each weekly reading of tanks
A, B, and C and then checked with Fisher's pairwise comparison. Graphs were made using Excel. The overall growth
for the experiment was measured by averaging the combined sizes of the seahorses from every weekly reading and
plotting the results of the five week experiment on excel.
4. Results
Survival in tanks A, B, and C at the conclusion of the experiment was a mere 33%. Tank A had two deaths in the
third and fourth weeks of the experiment, while tank B had one death after the third week, and one again in the
sixth week. Tank C had the best survivorship with no deaths until the sixth week of the experiment.
The standard length of the newly born seahorses was not significantly different (F=2.33, P=0.178) (figure 1). Over
the course of the experiment, the seahorses in tanks A and B grew considerably larger than in tank C. By the second
week there was statistically significant differences in standard length between tanks A, B, and C (F=24.5, P<0.001)
(figure 2 & 3). By the fourth week we could no longer do statistical analysis due to low sample size. At the
conclusion of the experiment the seahorses reared in tank A were 20% larger in length than in tank (figure 4).

Figure 1: Average Standard Length of first week (Nov. 1) of measurements. (F=2..33, P=0.178)
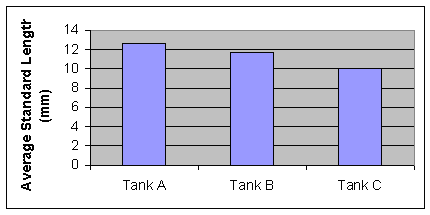
Figure 2: Average Standard Length of second week (Nov. 8) of measurements. (F=24.5, P<0.001) Growth rates begin to differ with statistical significance.
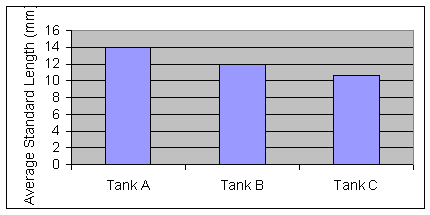
Figure 3: Average Standard Length of third week (Nov. 15) of measurements. (F=40.00, P<0.002)
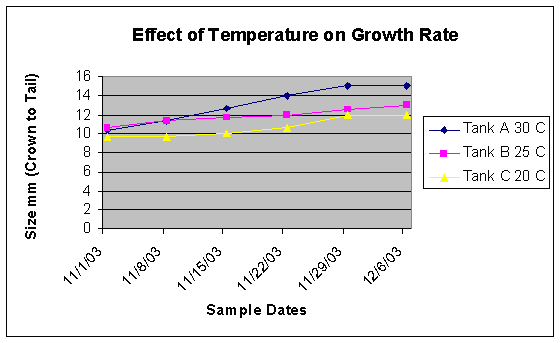
Figure 4: Over the course of the experiment seahorses in Tank A grew at a more rapid rate than in Tanks B, and
C.
5. Discussion
In the wild Hippocampus Zosterae is accustomed to temperatures between 20 ºC and 25 ºC. Tanks B, and
C lay within this threshold and had considerably better survival rates than tank A. Although the seahorses in tank
A grew quicker, their greater metabolic rate due to the increased temperature made them more stressed and prone
to disease. The major hurdle in all aquaculture industries is the ability to maintain good water quality. Although
ammonia, pH, and salinity were kept in proper ranges the lack of advanced filtration, vacuum, and circulation systems
created a higher potential for disease. There were many stressors in this experiment, due to its methods, which
could have reduced the survivorship of the seahorses as well. This includes the biweekly water changes that introduced
a flush of saltwater, which led to drastic variances in water temperature, especially in tank A. The weekly measurements
done by hand caused stress and also exposed the seahorses to disease. Both of theses stressors could have been
avoided by the addition of constant water circulators, as well as digital imaging to measure the length of the
seahorses.
Although the results of this experiment were statistically significant, they could have been improved by increasing
the sample size and replicates of each tank. This could increase our knowledge for the optimal temperature between
30 - 25 ºC that would increase survivorship, and excel growth rate. Also, a greater sample size would allow
us to create a cost/benefit analysis of higher temperatures on growth rate from a business prospective. Does the
benefit of reaching market size quicker eclipse the cost of low survivorship? In this experiment, we could see
that the two dominant markets for seahorses would require two different methods of aquaculture. That is, the medicinal
trade would need high quantities of seahorses quickly and hence tank A would supply that demand. However, the hobby
trade would demand healthy individuals, which would require a low stress habitat, which tanks B or C could provide.
The high growth rate, but low survivability of this experiment makes it difficult to assess the probable success
of seahorse aquaculture. The high prices associated with the seahorse trade give incentives to research factors
of which result in optimal growth rates, such as temperature, stocking densities, and food types (Vincent, 1996).
Such developments would increase the profitability of aquaculture, which would provide a sustainable alternative
for fishermen, and conserve the natural population of Hippocampus Zosterae.
Acknowledgements
I would like to thank Emily Doe and Prof. Fennessey for their help in taking care of the seahorses.
References
CITES, 2001. http://www.cites.org . Visited 12/06/03
Giwojna, P., Giwojna, B., 1999. Seahorse breeding secrets: Part I Ten common mistakes and how to avoid them. Freshwater
Mar. Aquar., January, 8-31
Giwojna, P., Giwojna, B., 1999. Seahorse breeding secrets: Part II Ten common mistakes and how to avoid them. Freshwater
Mar. Aquar., January, 8-31
James, P., Woods, C. 2001. Rearing Seahorses: Does Temperature Matter? Aquac. Update 9-10
Lawrence, C., 1998. Breeding Seahorses - Facts and fallacies. West Fish., Autumn, 39-40
Strawn, K., 1958. Life history of the Pygmy Seahorse, Hippocampus Zosterae. Jordan and Gilbert, at Cedar Key, Florida.
Vincent, A.C.J., 1996. The International Trade in Seahorses. TRAFFIC International, Cambridge, 163 pp.
Back to Aquaculture
Look at picturse of my Pygmy seahorses here