What is gene
therapy?
The aim of gene therapy
is to modify the genetic material of living cells for therapeutic purposes
(Amado and Chen, 1999). Gene therapy involves
the insertion of a functional gene or another molecule that contains and
information sequence into a cell to achieve a therapeutic effect.
Thus, the gene serves as a drug (Lasic, 1997).
There are two types of gene therapy: somatic cell and germ line.
Somatic cell gene therapy is the only technique now in use. The purpose
of the procedure is to eliminate the clinical consequences of a disease
and the inserted gene is not passed on to the patient's offspring.
In germ line gene therapy a healthy gene is inserted into the fertilized
egg of an animal that has a genetic effect. Every cell that develops
from this egg, including the reproductive cells, will have the new gene.
However, there are very serious social and ethical considerations with
this type of gene therapy (Nichols, 1998).
Before 1996 scientists relied mainly on modified
retroviruses
such as Moloney murine leukemia virus when gene transfer
into the chromosomes of target cells was needed, and adenovirus
vectors when such integration was not needed. However, there has
been little success in gene transfer with such virus vectors because even
though the vectors can enter into their target cells, the cells need to
be dividing, so that their nuclear membrane are broken down, for the gene
to enter and integrate into the chromosome (Sikorski
and Peters, 1998;
CFAR at UC San Diego).
However, scientists soon realized that members of the subfamily lentivirus,
such as the retrovirus human immunodeficiency virus (HIV),
would have the same ability to transfer genetic material into the genomes
of cells, but could do this with non-dividing, dormant cells in vivo and
growth-arrested cells in vitro (Amado and Chen, 1999;
CFAR
at UC San Diego). Exploring this new method of gene therapy has
been the work of many labs in the past few years.
Back to Index
What are lentiviral vectors?
Lentiviral vectors are a type
of retrovirus that can infect both dividing and nondividing cells because
their preintegration complex (virus “shell”) can get through the intact
membrane of the nucleus of the target cell. Lentiviruses can be used
to provide highly effective gene therapy as lentiviruses can change the
expression of their target cell's gene for up to six months. They
can be used for nondividing or terminally differentiated cells such as
neurons, macrophages, hematopoietic stem cells,
retinal photoreceptors, and muscle and liver cells, cell types for which
previous gene therapy methods could not be used. HIV is a very effective
lentiviral vector because it has evolved to infect and express its genes
in human helper T cells and other macrophages.
The only cells lentiviruses cannot gain access to are quiescent cells (in
the G0 state) because this blocks the reverse transcription
step (Amado and Chen, 1999). To understand how
HIV is a good vector for gene therapy, we must understand the structure
of HIV and how it functions and infects its host.
Structure of HIV
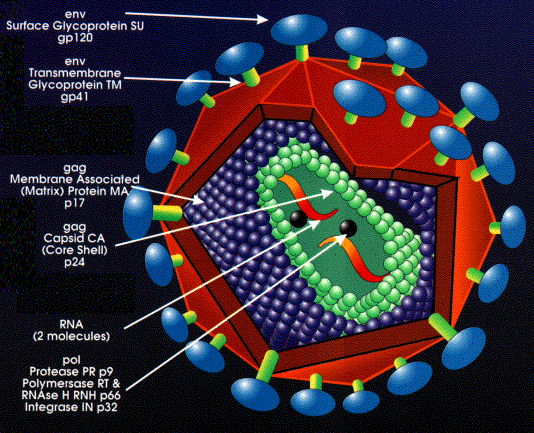
Structure
of HIV
Back to Index
Why HIV is a good vector for gene
therapy?
The preintegration complex of the human immunodeficient
virus (HIV), which allows the vector assess inside human cells, dividing
or non-diving, is composed of the enzyme integrase, the product of the
vpr
gene (an accessory gene), and a protein encoded by the gag gene
(an essential structural gene) called matrix. This matrix protein
contains a localization sequence which is recognized by the import machinery
of the nucleus of a cell. The virus is surrounded by a lipid bilayer
with protruding membrane proteins. One of these proteins, gp120,
is recognized by the host helper T cell CD4 receptor
protein. Then HIV binds to a secondary receptor (CCR5 or CXCR4)
and triggers a membrane fusion-mechanism with the gp41 transmembrane protein.
This allows the virus asses to the cell interior and the virus content
is released into the cytoplasm of the cell (Adler, Gifford,
and Sumner;
Schmidt, The HIV Page).
Once inside of the cell in the cytoplasm, the matrix protein of the HIV
contains a localization sequence that is recognized by the nuclear import
machinery, which docks the complex at a nuclear membrane pore. This
enables the preintegration complex of the HIV lentiviral vector to pass
into the nucleus (Amado and Chen, 1999).
-
Components of HIV Provirus
It is useful to understand the components of HIV and
how it affects its host cell. The major protein components of the
HIV virus can be seen in Table 1.
Table 1: The major protein components, which are expressed by
all retroviruses and are necessary for virus replication. They are encoded
by three major transcripts: gag, pol, env. These proteins
are synthesized as fusion proteins, which are post-translationally cleaved
by the virus-encoded protease. HIV has some additional genes (from Schmidt,
The HIV Page).
Name: Protein:
Fuction:
MA
Matrix
Matrix protein (gag gene); lines envelope
CA
Capsid
Capsid protein (gag gene); protects the core; most
abundant protein in virus particle
NC
Nucleocapsid
Capsid protein (gag gene); protects the genome;
forms the core
PR
Protease
Essential for gag protein cleavage during maturation
RT
Reverse transcriptase Reverse transcribes the RNA
genome; also has
RNAseH activity
IN
Integrase
Encoded by the pol gene; needed for integration of
the provirus
SU
Surface glycoprotein The outer envelope
glycoprotein; major virus
antigen
TM
Transmembrane protein The inner component of the mature envelope
glycoprotein
-
How HIV Infects Its Target Cell
Lentiviruses are the only type of virus that are diploid;
they have two strands of RNA. Thus, HIV contains a diploid single
stranded positive sense RNA-genome that is approximately 10 kb long.
The ends are flanked with long terminal repeats (LTRs).
A Psi-sequence is found near the 5’ end of the RNA-genome
which is necessary for packaging viral RNA into virus capsids to continue
the infection of HIV in its host (Schmidt, The HIV
Page). However, the HIV’s genetic information is integrated into
the DNA of the host cell, so its RNA must be converted into DNA inside
of the host for viral replication to be successful. This is done
by reverse transcription of the RNA into DNA, and some of the proteins
described in Table 1 are essential for this process. Reverse transcriptase
synthesizes the first strand of DNA from the RNA template, and the host
DNA polymerase synthesizes the second strand to produce dsDNA. Thus,
quiescent cells do not have the ability to perform this second step in
the reverse transcription process, so the RNA is not turned into DNA in
cells in the G0 state. This is the reason for the limitation on gene
therapy with HIV vectors. The DNA copy just made, which contains
the genes gag, env, and pol, is inserted by integrase
into the host genome (Adler, Gifford, and Sumner).
LTRs are also necessary for integration of the dsDNA into the host chromosome.
LTRs also serve as part of the promoter for transcription of the viral
genes (Schmidt, The HIV Page). Thus, the
virus is protected from attack by the immune system. It is this ability
of the HIV to integrate its genetic material into a host cell which scientists
would like to harness to put towards gene therapy. It has been shown
that the HIV vector has an even higher rate of expression in its hosts
cells than other retroviruses. HIV gene therapy vectors also do not
trigger immune reactions, making them very attractive delivery systems
(Adler, Gifford, and Sumner).
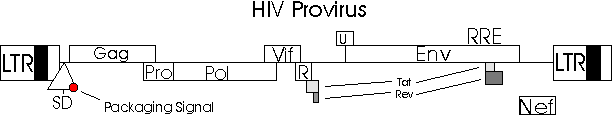
HIV
Provirus Used to Construct HIV Based Gene Therapy Vector
With the new genes from the HIV vector, DNA copy
duplication, excision, and integration of the virus can take place.
After infection and integration of the virus into its host regulatory proteins
let the retroviral DNA exist in three stages—the latent period with inactivity,
the stage where the virus gradually infects helper T cells, and then rapid
production of infective viral particles that are released into the blood
by the host cell lysis to infect other cells (Adler, Gifford,
and Sumner). Researchers must curtail these second and third
phases of HIV infection or HIV cannot be used as a gene therapy vector
as patients would be infected with not only the therapeutic gene product
but also the AIDS disease.
Back to Index
How are HIV lentiviral vectors
made?
To obtain a lentiviral gene therapy vector, a reporter
gene or therapeutic gene is cloned into a vector sequence that is flanked
by LTRs and the Psi-sequence of HIV. The LTRs are necessary to integrate
the therapeutic gene into the genome of the target cell, just as the LTRs
in HIV integrate the dsDNA copy of the virus into its host chromosome.
The Psi-sequence acts as a signal sequence and is necessary for packaging
RNA with the reporter or therapeutic gene in virions. Viral proteins which
make virus shells are provided in the packaging cell line, but are
not in context of the LTRs and Psi-sequences and so are not packaged into
virions. Thus, virus particles are produced that are replication
deficient, so are designed to be unable to continue to infect their host
after they deliver their therapeutic content (Schmidt,
HIV as a Vector for Gene Therapy).
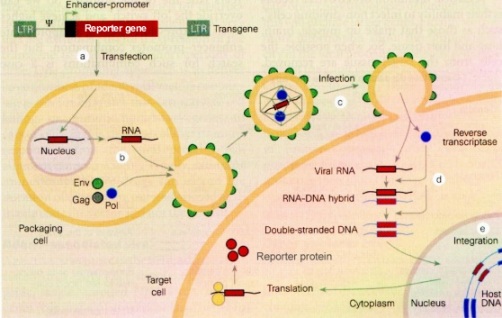
HIV
based gene therapy vector
-
HIV Vectors Have A Three Plasmid Expression System
Lentiviral vectors are usually created in a transient
transfection system in which a cell line is transfected with three separate
plasmid expression systems. These include the transfer
vector plasmid ( portions of the HIV provirus), the packaging
plasmid or construct, and a plasmid
with the heterologous envelop gene (ENV) of a different virus
(Amado and Chen, 1999). The three plasmid components
of the vector are put into a packaging cell which is then inserted into
the HIV shell (Kalapana, 1999). The virus
portions of the vector contain insert sequences so that the virus cannot
replicate inside the cell system (Adler, Gifford, and
Sumner).
Transfer Vector Plasmid
The transfer
vector plasmid contains cis-acting genetic sequences necessary for
the vector to infect the target cell and for transfer of the therapeutic
(or reporter) gene and contains restriction sites for insertion of desired
genes. The 3’ and 5’ LTRs, the original envelop proteins, and gag
sequence promoter have been removed (Adler, Gifford, and
Sumner; Naldini et al., 1996).
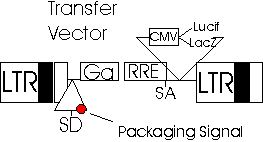
Transfer
Vector
Packaging Plasmid
The packaging
plasmid is the backbone of the virus system and is also known as
pCMVAR9. In this plasmid are found the elements required for vector
packaging such as structural proteins, HIV genes (except the gene env which
codes for infection of T cells, or the vector would only be able to infect
these cells), and the enzymes that generate vector particles (Amado
and Chen, 1999). Also contained is the human cytomegalovirus
(hCMV) which is responsible for the expression of the virus proteins during
translation. The packaging signals and their adjacent signals are
removed so the parts responsible for packaging the viral DNA have been
separated from the parts that activate them. Thus, the packaging
sequences will not be incorporated into the viral genome and the virus
will not reproduce after it has infected the host cell (Adler,
Gifford, and Sumner; Naldini, 1996).
Previous HIV vectors used two plasmids as the packaging plasmid contained
the viral envelop gene. However, in the newer, better vectors the
packaging plasmid lacks a viral envelop gene because this has been shown
to be more desirable in terms of titer (minimum volume needed to cause
a particular result in titration), stability, and broad range of target
cells (CFAR at UC San Diego).
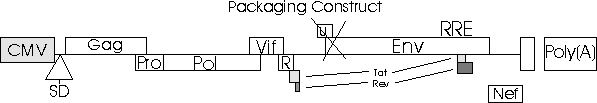
Packaging
Construct pCMVAR9
Envelop Gene of Third Plasmid
The third
plasmid’s envelope gene of a different virus specifies what type
of cell to target and infect instead of the T cells (Amado
and Chen, 1999). Normally HIV can infect only helper T-cells
because they use their gp120 protein to bind to the CD4 receptor.
However, it is possible to genetically exchange the CD4 receptor-binding
protein for another protein that codes for the different cell type on which
gene therapy will be performed (Schmidt, HIV
as a Vector for Gene Therapy). This gives the HIV lentiviral
vector a broad range of possible target cells. There are two types
of heterologous envelope proteins. The amphoteric envelop of MLV,
another type of vector, is transcribed first followed by the transcription
of the G glycoproteins of the vesicular stomatitis virus, known as VSV-G.
Both of these help to provide stability to the vector by bringing together
the particles that were made by the packaging plasmid pCMVAR9 (Adler,
Gifford, and Sumner; Naldini, 1996).
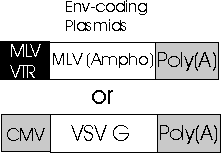
Envelop
Genes in the Third Plasmid
Scientists are challenged when making efficient packaging
lines of HIV gene therapy vectors because expression of the VSV-G envelope
and a number of HIV proteins is toxic to cells. They are dealing
with this problem by designing vectors whose expression of the packaging
genes and VSV-G can be turned on at will. Thus, the toxic genes can
be turned off to produce more vectors without toxicity. This cell
line can produce high titer vector without generating HIV vectors that
can self-replicate and infect the patient with disease (Amado
and Chen, 1999).
Back to Index
How are HIV lentiviral vectors
used?
-
Delivery Into Patients' Target Cells
The HIV-based vector can be delivered directly into
the body without
in vitro manipulations of the
patient’s cells (Adler, Gifford, and Sumner).
Additionally, lentiviral vectors have been shown to be superior to murine
retroviral vectors. Ex vivo manipulations
that activate stem cells with growth factors to induce cell division must
be carried for the retrovirus to be able to enter the stem cells.
However, it has been shown that ex vivo stem cell stimulation is
not necessary with lentiviral vectors, so the vectors can be inserted directly
into the patient and will find their way to the target cell (Amado
and Chen, 1999).
Previous gene therapy using retroviral vectors required
that cells be dividing, limiting therapy to proliferating cells in
vivo or ex vivo. In the ex vivo method, the
target cells are removed from the patient's body, treated to stimulate
replication and then transduced with the vector before being returned to
the patient. However, with lentiviral vectors there is no need for
ex
vivo treatment, and the target cells need not be dividing. The
HIV-based vector is simply injected into a patient, upon which it seeks
out its target cells based on cell membrane receptor proteins. Immune
responses to the lentiviruses have not been found (Peel,
1998).
-
Uses for HIV Lentiviral Vectors
Scientists have recently been using the HIV lentiviral
vector to repair neurons. HIV is also being developed as a delivery
system to provide successful gene therapy in many diseases such as metabolic
diseases, cancer, viral infection, cystic fibrosis,
muscular
dystrophy, hemophilia,
retinitis
pigmentosa, and maybe even Alzheimer’s disease
(Adler, Gifford, and Sumner; Naldini
et al.; Amado and Chen, 1999; Planelles).
-
Concerns With Using HIV Lentiviral Vectors
There is still concern with using lentiviral vectors
for safety reasons. One concern involves the possibility that the
HIV could self-replicate and could be produced during manufacture of the
vector in the packaging cell line or in the target cells by a process of
recombination. Thus, the person undergoing gene therapy would also
be infected with HIV in addition to the new therapeutic gene. A self-replicating
infectious vector could cause cancer by inserting itself into the host
genome and activate a neighboring proto-oncogene, thus
causing mutagenesis (Amado and
Chen, 1999).
Back to Index
Current research involving lentiviral
vectors
Because scientists have shown that lentiviruses,
such as HIV, are successful and efficient gene delivery vehicles, the field
has now turned its attention to producing vectors with built-in safety
features to prevent the development of replication competent lentiviruses
(RCL). However, even the earliest studies with
HIV lentiviral vectors did not generate RCL in vitro or in vivo (Amado
and Chen, 1999), but precautions are still very important.
HIV lentiviral vectors are being produced whose packaging
plasmid does not contain the necessary HIV genes. This does not interfere
with efficient vector production and is a great increase in safety because
potential RCL’s cannot use the HIV genes necessary for replication of HIV
in humans. The drawback to these vectors is that they cannot transduce
macrophages because the accessory gene vpr is needed for HIV infection
of this type of cells. Thus, scientists are showing that the type
of lentiviral vector necessary is dependent on the type of cell chosen
as target, so the HIV vectors will be made with different accessory genes
(Amado and Chen, 1999).
Researchers at the Salk Institute are creating HIV
lentiviral vectors that are self-inactivating. The scientists are
working on packaging a defective HIV genome that contains only the necessary
elements for gene transduction into a virion that has a broad host range.
HIV normally targets human CD4 (helper T cells) through interactions with
membrane-bound target proteins, but to broaden the host cell targets a
surrogate targeting molecule (VSV-G) was inserted into the viral membrane.
The HIV genome was modified to produce a minimal construct and the cytomegalovirus
promoter and green fluorescence protein as a marker were added (Sikorski
and Peters, 1998). A deletion in the LTR region at the end of
the virus genome is also created. These are unique cis-acting sequences
that are essential to the virus life cycle. The deletion inactivates
the LTR promoter and eliminates the production of vector RNA. The
gene to be transferred by the vector is expressed from an exogenous viral
or cellular promoter that is inserted into the lentiviral vector.
Inactivation of the promoter activity of the LTR reduces the possibility
of insertional mutagenesis as the lentiviral products are integrated into
the host genome. Also, as expression of the vector RNA is eliminated,
the potential for RCL production in the target cell is further minimized
(Amado and Chen, 1999).
Other safety methods include using specific internal
promoters that regulate gene expression either temporally or with tissue
or cell specificity so as to prohibit gene expression that would cause
replication of HIV in the gene therapy target cell (Amado
and Chen, 1999).
-
Use of Non-Human Lentiviral Vectors
By using non-human lentiviruses, scientists hope to
bypass the issue of host infection by the gene therapy vector. Researchers
are developing non-human lentiviruses such as the feline immunodeficiency
virus (FIV) to be used in gene therapy (Amado
and Chen, 1999). FIV infects two to twenty percent of domestic
cats worldwide and causes a disease similar to human AIDS. While
humans have been exposed to this virus through cat bites, humans have never
been shown to be infected by the virus. It has been shown that evolutionarily
FIV diverged early on from HIV and other lentiviruses. Researchers
at the University of San Diego, though, have found that while nonprimate
lentiviruses may provide safer alternatives to HIV they have highly restricted
host range of infection. However, promoter substitution of FIV enabled
an env-deleted, three plasmid, human cell-FIV lentiviral vector system
to express high levels of FIV proteins and FIV vectors in human cells.
The researchers replaced the U3 element within the 5’ LTR of FIV with the
human cytomegalovirus early gene promoter. Pseudotyped FIV vectors
were shown to be able to efficiently transduced dividing, growth-arrested,
and postmitotic human targets. The researchers also showed that human
cells supported mechanisms of the FIV life cycle needed for efficient lentiviral
vector transduction. It is the U3 element in FIV that is the only
restriction to the productive phase of FIV replication in human cells.
The researchers concluded that lentivirus-specific properties of FIV vectors
are retained in human cells, and they speculate that eventually FIV vector
will have advantages in human clinical use. Additionally, vectors
derived from FIV may represent a safer alternative to HIV vectors, even
those with deleted nonstructural proteins, because they cannot induce HIV-reactive
antibodies in recipients. Overall, FIV has experimental advantages
over HIV (Poeschla, Wong-Staal, and Looney, 1998).
Researchers at the University of North Carolina at
Chapel Hill are working with equine infectious anemia virus (EIAV)
to be used as a lentiviral vector in humans. EIAV is a lentivirus
that normally infects horses, donkeys, and mules. It has been shown
to be able to infect mature macrophages, and thus has the potential to
infect quiescent cells, and has relatively simple genome organization.
The researchers constructed separate plasmids encoding EIAV proteins, a
viral envelop, and an EIAV vector. They attempted to broaden the
host range of the vector to human cells by using non-EIAV enhancer/promoter
elements to drive expression and a non-EIAV envelop glycoprotein.
They succeeded in transducing up to about 60 to 70 percent of human CFT1
cells which were placed in a culture dish. This is still quite a
bit lower than the transduction level obtained using murine retroviruses,
but more work with EIAV will hopefully increase the efficiency of this
procedure. In addition, the fact that both EIAV-based and HIV vector
can mediate gene transfer and expression to non-dividing human cells suggests
that nuclear targeting mechanisms of equine and human lentiviruses are
functionally conserved (Olsen, 1998).
-
Lentiviral Vectors for Hematopoietic Stem Cells
Many recent studies with lentiviral vectors have focused
on modifying the hematopoietic stem cell which has the capacity to self-renew
and to differentiate into all of the mature cells of the blood and immune
systems. Thus, by introducing therapeutic genes into stem cells many
diseases that affect these systems could be treated (Amado
and Chen, 1999).
-
Gene Therapy for Cystic Fibrosis
Researchers at the Institute for
Gene Therapy at the University of Pennsylvania evaluated a replication-deficient
vector based on HIV for gene transfer directly into the lung to correct
the genetic defects of cystic fibrosis (CF). They expanded the target
range of the vector by adding the vesticular stomatitis virus G protein
into the HIV vector envelop. LacZ was the reporter
gene in the HIV-based vector, so the level of transduction was assessed
based on the expression of lacZ. The researchers were successful
at transducing nondividing airway epithelial cells in vitro, whereas they
were unsuccessful when using murine-based retroviral vectors. Thus,
the vector corrected the CF defect in proliferating airway cells.
There were complications with differentiated epithelial lung cells as the
vectors did not effectively transduce these cells. The blockage appeared
to be at the level of entry, the researchers reported. Further experimentation
is being conducted to examine the problems of cell entry into differentiated
cells (Goldman, et al., 1997).
-
Liver-Directed Gene Therapy
Initial research aimed at delivering
genes to the liver in vivo with HIV-based lentiviral vectors showed promising
results, reported Ganjam Kalpana of Albert Einstein College of Medicine
this year. This scientist developed a crippled version of HIV and
used it as a vehicle for
in vivo gene therapy on low-density lipoprotein
receptor-deficient Watanabe heritable hyperlipidemic rabbits. A eukaryotic
humanized gene fluorescent protein gene was cloned into the transfer vector
to act as the reporter gene for successful cell transduction. The
HIV vector was highly superior to previous methods of gene therapy using
retroviral vectors which were highly invasive to the patient. There
was also no host mediated cellular immune response to the lentiviral vector
(Kalpana, 1999). This is another application
to HIV-based gene therapy vectors that has been shown to be successful.
-
Therapy Against Retinitis Pigmentosa
Retinitis pigmentosa is an inherited genetic disease
which causes the retina to degenerate leading to loss of visual field and
night blindness. Genetic defects of photoreceptor cells of the visual
system are the cause of this disease. A vector for gene therapy of
retinitis pigmentosa should only target photoreceptor cells, which are
located in the outer nuclear layer of the retina. Miyoshi, Takahashi,
Gage, and Verma conducted an experiment using an HIV-based vector with
a gfp-gene (green fluorescent protein) as a reporter. The vector
was injected into rat retina. It was shown that the HIV-based vector
did achieve long-term gene expression in the photoreceptor cells when a
rhodopsin-promoter was used in the vector. This is only active in
the photoreceptor cells, so the vector only targets these cells and not
others in the retina. Thus, the researchers were successful in performing
gene therapy on their rat patients (Schmidt,
HIV as a Vector For Gene Therapy).
Back to Index
References:
Adler,
K., J. Gifford, and R. Sumner. HIV as a Vector in Gene Therapy.
[Online.]
http://wwwpp.uwrf.edu/%7Ekk00/hivvector/hivvector.htm.
[12-13-99, last date accessed.]
Amado,
R. G. and YI. S.. Chen. 1999. Lentiviral Vectors—the Promise
of Gene Therapy Within Reach? Science. 285
(5428): 674-76.
CFAR:
Center for AIDS Research at UC San Diego. Last update 10-18-99.
Lentiviral Vector Core. [Online.]
http://hsrd.ucsd.edu/Cfar/lenti/lenti.html.
[12-13-99, last date accessed.]
Goldman,
M. J., P. Lee, J. Yang, and J. M. Wilson. 1997. Lentiviral
Vectors for Gene Therapy of Cystic Fibrosis.
Human Gene Therapy. 8: 2261-2268.
Kalpana,
G. V. 1999. Retroviral Vectors for Liver-directed Gene
Therapy. Seminar in Liver Disease. 19 (1): 27-37.
Lasic, D. D. Liposomes in Gene Delivery.
New York: CRC Press, 1997.
Naldini
et al. 1997. Lentiviral Vectors for in Vivo Gene Delivery.
The International Symposium on Gene Therapy for
Hemophilia. [Online.] http://www.med.unc.edu/wrkunits/3ctrpgm/thromb/naldini.htm.
[12-13-99, last date accessed.]
Naldini
et al. 1996. In Vivo Gene Delivery and Stable Tranduction
of Nondividing Cells by a Lentiviral Vector. Science.
272: 263-267.
Nichols, E. K. Human Gene Therapy.
Cambridge, Massachusettes: Harvard University Press, 1998.
Olsen, J. C. 1998. Gene Transfer Vectors
Derived From Equine Infectious Anemia Virus. Gene Therapy.
5: 1481-1487.
Peel,
David. 1998. Retroviral Vectors and Lentiviral Vectors.
Department of Microbiology & Immunology, University of
Leicester. [Online.] http://science.uniserve.edu.au/mirror/microbiol/335/peel/peel2.html.
[12-13-99, last date accessed.]
Poeschla,
E. M., F. Wong-Staal, and D. J. Looney. 1998. Efficient
Transduction of Nondividing Human Cells by Feline
Immunodeficiency Virus Lentiviral Vectors.
Nature Medicine. 4 (3): 354-357.
Planelles,
V. 1999. Homepage of Vicente Planelles. [Online.]
http://www.urmc.rochester.edu/gebs/faculty/Vicente_Planelles.htm.
[12-13-99, last date accessed.]
Sikorski,
R. and R. Peters. 1998. Gene Therapy: Treating with HIV.
Science. 282 (5393): 1438a.
Schmidt,
Uli. The HIV Page. [Online.] http://bioinformatik.biochemtech.uni-halle.de/uli/genetherapy/hiv.htm.
[12-13-99,
last date accessed.]
_____.
HIV as a Vector for Gene Therapy. [Online.]
http://bioinformatik.biochemtech.uni-halle.de/uli/genetherapy/genehiv.htm.
[12-13-99, last date accessed.]
Tighe,
R. and J. Fritz. 1996. Lentiviral Vectors (HIV-based).
[Online.] http://www.mc.vanderbilt.edu/gcrc/gene/hiv.htm.
[12-13-99, last date accessed.]
Back to Index
Glossary:
Adenovirus: another early vector in gene
therapy; used when gene transfer into the chromosomes of target cells was
not needed
Alzheimer’s disease: a disease marked by progressive
loss of mental capacity resulting from degeneration of the brain cells
CD4: a membrane protein of helper T cells
that interacts with membrane proteins of HIV
Cystic fibrosis: a hereditary disease of the
exocrine glands, usually developing during early childhood and affecting
mainly the pancreas, respiratory system, and sweat glands; characterized
by the production of abnormally viscous mucus by the affected glands, usually
resulting in chronic respiratory infections and impaired pancreatic function
EIAV: equine infectious anemia virus; a lentivirus
that normally infects horses, donkeys, and mules with anemia
Ex vivo: out of the patient’s body
FIV: feline immunodeficiency virus; infects
two to twenty percent of domestic cats worldwide and causes a disease similar
to human AIDS
Gene therapy: involves the insertion
of a functional gene or another molecule that contains and information
sequence into a cell to achieve a therapeutic effect
gp120: a membrane protein of HIV that interacts
with membrane proteins of its target cell
Helper T cells: components of the human
immune system and the target cells of HIV
Hemophilia: several hereditary blood-coagulation
disorders in which the blood fails to clot normally because of a deficiency
or an abnormality of one of the clotting factors; a recessive trait associated
with the X-chromosome so manifested almost exclusively in males
HIV: human immunodeficiency virus; a virus
of the human immune system that causes the AIDS disease
In vitro: out of the patient’s body in a test
tube or culture dish
In vivo: in the patient’s body
Lentiviral vectors: Lentiviruses are a type
of retrovirus that can infect both dividing and nondividing cells because
their preintegration complex (virus “shell”) can get through the intact
membrane of the nucleus of the target cell
LTR: long terminal repeats; flank the ends
of the HIV genome and contain a Psi-sequence near the 5’ end of the RNA-genome
Macrophages: any of the large phagocytic
cells of the reticuloendothelial system
Moloney murine leukemia virus: a retrovirus
that was used in early gene therapy experiments; used when gene transfer
into the chromosomes of target cells was needed
Muscular dystrophy: a group of progressive
muscle disorders caused by a defect in one or more genes that control muscle
function and characterized by gradual irreversible wasting of skeletal
muscle
Mutagenesis: formation or development of
a mutation
Proto-oncogene: a normal gene that could develop
into one that causes a transformation of normal cells into cancerous tumor
cells, especially a viral gene that transforms a host cell into a tumor
cell
Psi-sequence: located at the 5’ end of the
HIV’s LTR; is necessary for packaging viral RNA into virus capsids to continue
the infection of HIV in its host
RCL: a replication competent lentivirus; an
HIV lentivirus that can infected its host with the AIDS disease
Retinitis pigmentosis: an inherited genetic
disease which causes the retina to degenerate leading to loss of visual
field and night blindness; caused by genetic defects of photoreceptor cells
of the visual system are the cause of this disease
Retroviruses: a class of viruses
Reporter gene: inserted into a genome
along with a new gene to show the position and existence of the new gene
Reverse transcription: the process of converting
RNA to DNA



