|
| "Inadequate
dosing of antibiotics is probably an important reason for misuse and subsequent
risk of resistance. A recommendation on proper dosing regimens for
different infections would be an important part of a comprehensive strategy.
The possibility to produce such a dose recommendation based on pharmacokinetic
and pharmcodynamic considerations will be further investigated in one of
the CPMP working parties..."
- European Agency of the Evaluation of Medicinal Products (London) Bambeke and Tulkens, 1999 |
Drug-resistant infectious microbes are an increasingly important public health concern that warrant many individuals and organizations on public and private sectors worldwide to work together: health care providers on the local, state, and national level, United States federal agencies, such as the NIH, CDC, and Food and Drug Administration, the World Health Organization, pharmaceutical companies, and academia (Fauci, 1999). Aside from the public health threat, the search for microbial-sensitive treatments is expensive and contributes to the higher costs of health care. The treatment regimes necessitate more expensive pharmaceuticals and longer hospital stays for individuals infected; in fact, the Institute of Medicine estimated that in the United States the annual cost of treating therapeutic resistant infections could be as high as $30 billion (NIAID, 2000). Genetic sequencing of pathogens with high-level resistance, including N. gonorrhoeae, HIV, and S. pyogenes, are being supported by the NIH, and has led to the development of molecular tools and further insights to drug design (Fauci, 1999).
In bacteria, antimicrobial resistance is faciliatated through their ability to quickly adapt to new environments, and along with their ability to replicate very quickly comes the aptitude to mutate their DNA or DNA acquired from another drug-resistant bacteria through transformation; this evolution furthers the adapted gene's predominance (NIAID, 2000). Also, the over-prescription and sometimes inappropriate use of antimicrobials compels the bacteria to develop resistance (Toebe, 2001). There are numerous socioeconomic factors that contribute to antibiotic resistance, as listed by Toebe, 2001:
Mechanisms of resistance
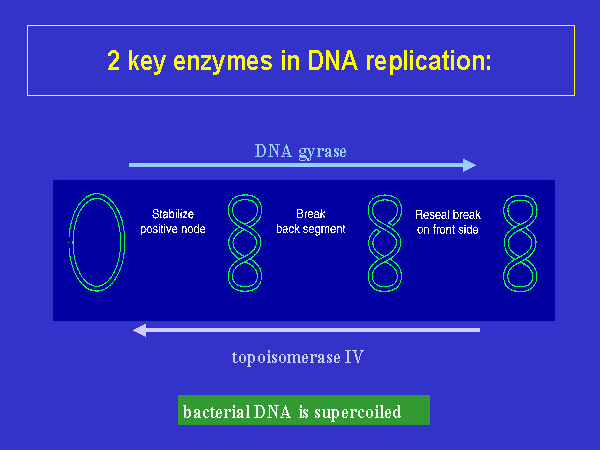
Fluoroquinolones work to inhibit bacterial DNA synthesis and they accomplish this through inhibiting the bacterial DNA gyrase (CTR, 1997). DNA gyrase is the enzyme responsible for negative supercoiling of double-stranded DNA that balances the positive supercoiling of DNA replication; without it, irreparable damages occur in the DNA strand (CTR, 1997). Topoisomerase IV initiates DNA decatenation, the mechanism by which two daughter chromosomes are separated after division of a bacterial chromosome (CTR, 1997).
One must understand how antimicrobial resistance develops before evaluating the implications of fluoroquinolone resistance. There are three mechanisms by which File and Slama, 2000 describe how bacteria can develop resistance:
There are two subunits
of DNA gyrase and Topoisomerase IV: gyrA and gyrB, and parC
and parE, respectively.

|
|
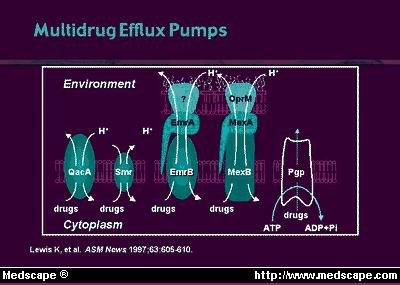
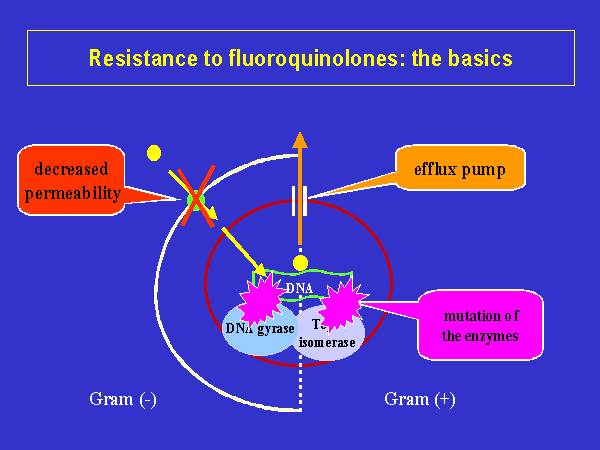
Bacterial
changes in permeation routes or the effects of efflux mechanisms that remove
fluoroquinolones from inside the bacterium (Figure 2), or both, can reduce
the access of the antibiotic, increasing resistance; whereas spontaneous
mutations in the chromosomal genes, and rare cases of plasmid-mediated
resistance promote bacterial resistance to quinolones (File
and Slama, 2000). A reduction in the porin diffusion channels
in the outer membrane of the Gram negative bacteria cell envelope, which
lead to the cytoplasmic membrane, or an increase in the efflux mechanism
activity, may decrease drug permeation or its ability to reach bacterial
targets
(Figure 3) (File and Slama, 2000). |
|
In 1998 (Ison et al.), a surveillance project in the United Kingdom established that ciprofloxacin, replacing penicillin, was still a highly effective agent in treating gonorrhea, but the identified drift in susceptibility most likely resulted from the increased usage of it. This drift was attributed to N. gonorrhoeae isolates exhibiting chromosomal and/or plasmid-mediated resistance to penicillin, thus using ciprofloxacin instead. Double mutations in gyrA (the DNA gyrase gene) have been correlated with high-level resistance (Kocagoz et al., 1996) in isolates with MIC's greater than 1mg/L, along with additional mutations in some isolates in the topoisomerase gene, parC.
Results of the
study indicated that almost half of the N. gonorrhoeae resistant
isolates were penicillinase producers, while
the rest displayed increased resistance to penicillin (Table
2); plasmid-mediated high-level resistance to tetracycline was not
observed (Ison et al., 1998).
Mutations in the quinolone resistance-determining region (QRDR) of the
gyrA
gene did result in ciprofloxacin resistance, and certified that through
a molecular-based technique, gyrA and parC could act as resistance
indicators. In Mycobacterium tuberculosis, Staphylococcus
aureus, and Streptococcus pneumoniae,
efflux mechanisms have
been associated with fluoroquinolone resistance (Kocagoz
et al., 1996), and through acquiring the mtrRCDE operon in transformants,
N.
gonorrhoeae's resistance to hydrophobic agents is mediated, but this
is not conclusive for ciprofloxacin resistance (Ison
et
al., 1998).